Explanations for the clinical and microscopic localization of lesions in pemphigus foliaceus and vulgaris
- PMID: 10021453
- PMCID: PMC408100
- DOI: 10.1172/JCI5252
Explanations for the clinical and microscopic localization of lesions in pemphigus foliaceus and vulgaris
Abstract
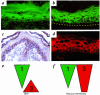
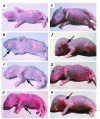
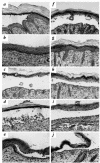
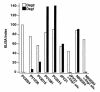
Patients with pemphigus foliaceus (PF) have blisters on skin, but not mucous membranes, whereas patients with pemphigus vulgaris (PV) develop blisters on mucous membranes and/or skin. PF and PV blisters are due to loss of keratinocyte cell-cell adhesion in the superficial and deep epidermis, respectively. PF autoantibodies are directed against desmoglein (Dsg) 1; PV autoantibodies bind Dsg3 or both Dsg3 and Dsg1. In this study, we test the hypothesis that coexpression of Dsg1 and Dsg3 in keratinocytes protects against pathology due to antibody-induced dysfunction of either one alone. Using passive transfer of pemphigus IgG to normal and DSG3(null) neonatal mice, we show that in the areas of epidermis and mucous membrane that coexpress Dsg1 and Dsg3, antibodies against either desmoglein alone do not cause spontaneous blisters, but antibodies against both do. In areas (such as superficial epidermis of normal mice) where Dsg1 without Dsg3 is expressed, anti-Dsg1 antibodies alone can cause blisters. Thus, the anti-desmoglein antibody profiles in pemphigus sera and the normal tissue distributions of Dsg1 and Dsg3 determine the sites of blister formation. These studies suggest that pemphigus autoantibodies inhibit the adhesive function of desmoglein proteins, and demonstrate that either Dsg1 or Dsg3 alone is sufficient to maintain keratinocyte adhesion.
Figures
References
-
- Stanley, J.R. 1993. Pemphigus. In Dermatology in general medicine. T.B. Fitzpatrick, A.Z. Eisen, K. Wolff, I. M. Freedberg, and K.F. Austen, editors. McGraw-Hill. New York, NY. 606–615.
-
- Lever, W.F. 1965. Pemphigus and pemphigoid. Charles C. Thomas. Springfield, IL. 15–66.
-
- Meurer M, Millns JL, Rogers RS, III, Jordon RE. Oral pemphigus vulgaris. A report of ten cases. Arch Dermatol. 1977;113:1520–1524. - PubMed
-
- Beutner EH, Jordon RE. Demonstration of skin antibodies in sera of pemphigus vulgaris patients by indirect immunofluorescent staining. Proc Soc Exp Biol Med. 1964;117:505–510. - PubMed
-
- Rappersberger K, Roos N, Stanley JR. Immunomorphological and biochemical identification of the pemphigus foliaceus autoantigen within desmosomes. J Invest Dermatol. 1992;99:323–330. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

