Lung fluid transport in aquaporin-1 and aquaporin-4 knockout mice
- PMID: 10021464
- PMCID: PMC408096
- DOI: 10.1172/JCI4138
Lung fluid transport in aquaporin-1 and aquaporin-4 knockout mice
Abstract
The mammalian lung expresses water channel aquaporin-1 (AQP1) in microvascular endothelia and aquaporin-4 (AQP4) in airway epithelia. To test whether these water channels facilitate fluid movement between airspace, interstitial, and capillary compartments, we measured passive and active fluid transport in AQP1 and AQP4 knockout mice. Airspace-capillary osmotic water permeability (Pf) was measured in isolated perfused lungs by a pleural surface fluorescence method. Pf was remarkably reduced in AQP1 (-/-) mice (measured in cm/s x 0.001, SE, n = 5-10: 17 +/- 2 [+/+]; 6.6 +/- 0.6 AQP1 [+/-]; 1.7 +/- 0.3 AQP1 [-/-]; 12 +/- 1 AQP4 [-/-]). Microvascular endothelial water permeability, measured by a related pleural surface fluorescence method in which the airspace was filled with inert perfluorocarbon, was reduced more than 10-fold in AQP1 (-/-) vs. (+/+) mice. Hydrostatically induced lung interstitial and alveolar edema was measured by a gravimetric method and by direct measurement of extravascular lung water. Both approaches indicated a more than twofold reduction in lung water accumulation in AQP1 (-/-) vs. (+/+) mice in response to a 5- to 10-cm H2O increase in pulmonary artery pressure for five minutes. Active, near-isosmolar alveolar fluid absorption (Jv) was measured in in situ perfused lungs using 125I-albumin as an airspace fluid volume marker. Jv (measured in percent fluid uptake at 30 min, n = 5) in (+/+) mice was 6.0 +/- 0.6 (37 degrees C), increased to 16 +/- 1 by beta-agonists, and inhibited to less than 2.0 by amiloride, ouabain, or cooling to 23 degrees C. Jv (with isoproterenol) was not affected by aquaporin deletion (18.9 +/- 2.2 [+/+]; 16.4 +/- 1.5 AQP1 [-/-]; 16.3 +/- 1.7 AQP4 [-/-]). These results indicate that osmotically driven water transport across microvessels in adult lung occurs by a transcellular route through AQP1 water channels and that the microvascular endothelium is a significant barrier for airspace-capillary osmotic water transport. AQP1 facilitates hydrostatically driven lung edema but is not required for active near-isosmolar absorption of alveolar fluid.
Figures

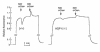
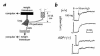


References
-
- Olver, R.E. 1994. Fluid secretion and absorption in the fetus. In Fluid and solute transport in the airspaces of the lung. R.M. Effros and H.K. Chang, editors. Marcel Dekker. New York, NY. 281–302.
-
- Matthay MA, Folkesson H, Verkman AS. Salt and water transport across alveolar and distal airway epithelia in the adult lung. Am J Physiol. 1996;270:L487–L503. - PubMed
-
- Wangensteen, O.D. 1994. Nonselective solute transport across the pulmonary epithelium. In Fluid and solute transport in the airspaces of the lungs. Vol. 70. R.M. Effros and H.K. Chang, editors. Marcel Dekker. New York, NY. 374–397.
-
- Hummler E, et al. Early death due to defective neonatal lung liquid clearance in αENaC-deficient mice. Nat Genet. 1996;12:325–328. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

