Leptin suppression of insulin secretion and gene expression in human pancreatic islets: implications for the development of adipogenic diabetes mellitus
- PMID: 10022436
- PMCID: PMC2927866
- DOI: 10.1210/jcem.84.2.5460
Leptin suppression of insulin secretion and gene expression in human pancreatic islets: implications for the development of adipogenic diabetes mellitus
Abstract
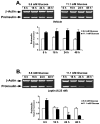
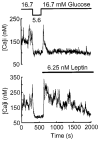
Previously we demonstrated the expression of the long form of the leptin receptor in rodent pancreatic beta-cells and an inhibition of insulin secretion by leptin via activation of ATP-sensitive potassium channels. Here we examine pancreatic islets isolated from pancreata of human donors for their responses to leptin. The presence of leptin receptors on islet beta-cells was demonstrated by double fluorescence confocal microscopy after binding of a fluorescent derivative of human leptin (Cy3-leptin). Leptin (6.25 nM) suppressed insulin secretion of normal islets by 20% at 5.6 mM glucose. Intracellular calcium responses to 16.7 mM glucose were rapidly reduced by leptin. Proinsulin messenger ribonucleic acid expression in islets was inhibited by leptin at 11.1 mM, but not at 5.6 mM glucose. Leptin also reduced proinsulin messenger ribonucleic acid levels that were increased in islets by treatment with 10 nM glucagon-like peptide-1 in the presence of either 5.6 or 11.1 mM glucose. These findings demonstrate direct suppressive effects of leptin on insulin-producing beta-cells in human islets at the levels of both stimulus-secretion coupling and gene expression. The findings also further indicate the existence of an adipoinsular axis in humans in which insulin stimulates leptin production in adipocytes and leptin inhibits the production of insulin in beta-cells. We suggest that dysregulation of the adipoinsular axis in obese individuals due to defective leptin reception by beta-cells may result in chronic hyperinsulinemia and may contribute to the pathogenesis of adipogenic diabetes.
Figures

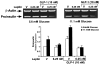
References
-
- Smith SR. The endocrinology of obesity. Endocrinol Metab Clin North Am. 1996;25:921–942. - PubMed
-
- Spiegelman BM, Flier JS. Adipogenesis and obesity: rounding out the big picture. Cell. 1996;87:377–389. - PubMed
-
- Bray GA. Obesity. In: Fauci AS, Braunwald E, Isselbacher KJ, Wilson JD, Martin JB, Kasper DL, Hauser SL, Longo DL, editors. Harrison’s principles of internal medicine. 1998. pp. 454–462.
-
- Cairney J, Wade TJ. Correlates of body weight in the 1994 National Population Health Survey. Int J Obes Relat Metab Disord. 1998;22:584–591. - PubMed
-
- Ramachandran A, Snehalatha C, Viswanathan V, Viswanathan M, Haffner SM. Risk of noninsulin dependent diabetes mellitus conferred by obesity and central adiposity in different ethnic groups: a comparative analysis between Asian Indians, Mexican Americans and Whites. Diabetes Res Clin Pract. 1997;36:121–125. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

