p50(cdc37) acting in concert with Hsp90 is required for Raf-1 function
- PMID: 10022854
- PMCID: PMC83960
- DOI: 10.1128/MCB.19.3.1661
p50(cdc37) acting in concert with Hsp90 is required for Raf-1 function
Abstract
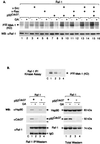
Genetic screens in Drosophila have identified p50(cdc37) to be an essential component of the sevenless receptor/mitogen-activated kinase protein (MAPK) signaling pathway, but neither the function nor the target of p50(cdc37) in this pathway has been defined. In this study, we examined the role of p50(cdc37) and its Hsp90 chaperone partner in Raf/Mek/MAPK signaling biochemically. We found that coexpression of wild-type p50(cdc37) with Raf-1 resulted in robust and dose-dependent activation of Raf-1 in Sf9 cells. In addition, p50(cdc37) greatly potentiated v-Src-mediated Raf-1 activation. Moreover, we found that p50(cdc37) is the primary determinant of Hsp90 recruitment to Raf-1. Overexpression of a p50(cdc37) mutant which is unable to recruit Hsp90 into the Raf-1 complex inhibited Raf-1 and MAPK activation by growth factors. Similarly, pretreatment with geldanamycin (GA), an Hsp90-specific inhibitor, prevented both the association of Raf-1 with the p50(cdc37)-Hsp90 heterodimer and Raf-1 kinase activation by serum. Activation of Raf-1 via baculovirus coexpression with oncogenic Src or Ras in Sf9 cells was also strongly inhibited by dominant negative p50(cdc37) or by GA. Thus, formation of a ternary Raf-1-p50(cdc37)-Hsp90 complex is crucial for Raf-1 activity and MAPK pathway signaling. These results provide the first biochemical evidence for the requirement of the p50(cdc37)-Hsp90 complex in protein kinase regulation and for Raf-1 function in particular.
Figures
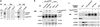





Similar articles
-
Hsp90 regulates p50(cdc37) function during the biogenesis of the activeconformation of the heme-regulated eIF2 alpha kinase.J Biol Chem. 2001 Jan 5;276(1):206-14. doi: 10.1074/jbc.M007583200. J Biol Chem. 2001. PMID: 11036079
-
Hsp90/p50cdc37 is required for mixed-lineage kinase (MLK) 3 signaling.J Biol Chem. 2004 May 7;279(19):19457-63. doi: 10.1074/jbc.M311377200. Epub 2004 Mar 4. J Biol Chem. 2004. PMID: 15001580
-
The hsp90-binding antibiotic geldanamycin decreases Raf levels and epidermal growth factor signaling without disrupting formation of signaling complexes or reducing the specific enzymatic activity of Raf kinase.J Biol Chem. 1997 Feb 14;272(7):4013-20. doi: 10.1074/jbc.272.7.4013. J Biol Chem. 1997. PMID: 9020108
-
[Molecular chaperone HSP90 as a novel target for cancer chemotherapy].Nihon Yakurigaku Zasshi. 2003 Jan;121(1):33-42. doi: 10.1254/fpj.121.33. Nihon Yakurigaku Zasshi. 2003. PMID: 12617036 Review. Japanese.
-
Regulation of RAF family kinases: new insights from recent structural and biochemical studies.Biochem Soc Trans. 2024 Jun 26;52(3):1061-1069. doi: 10.1042/BST20230552. Biochem Soc Trans. 2024. PMID: 38695730 Free PMC article. Review.
Cited by
-
The co-chaperone Cdc37 regulates the rabies virus phosphoprotein stability by targeting to Hsp90AA1 machinery.Sci Rep. 2016 Jun 2;6:27123. doi: 10.1038/srep27123. Sci Rep. 2016. PMID: 27251758 Free PMC article.
-
Bipartite Role of Heat Shock Protein 90 (Hsp90) Keeps CRAF Kinase Poised for Activation.J Biol Chem. 2016 Nov 18;291(47):24579-24593. doi: 10.1074/jbc.M116.746420. Epub 2016 Oct 4. J Biol Chem. 2016. PMID: 27703006 Free PMC article.
-
Heat shock protein expression in canine malignant mammary tumours.BMC Cancer. 2006 Jun 27;6:171. doi: 10.1186/1471-2407-6-171. BMC Cancer. 2006. PMID: 16803633 Free PMC article.
-
Activation of B-Raf kinase requires phosphorylation of the conserved residues Thr598 and Ser601.EMBO J. 2000 Oct 16;19(20):5429-39. doi: 10.1093/emboj/19.20.5429. EMBO J. 2000. PMID: 11032810 Free PMC article.
-
Cdc37p is required for stress-induced high-osmolarity glycerol and protein kinase C mitogen-activated protein kinase pathway functionality by interaction with Hog1p and Slt2p (Mpk1p).Eukaryot Cell. 2007 Mar;6(3):521-32. doi: 10.1128/EC.00343-06. Epub 2007 Jan 12. Eukaryot Cell. 2007. PMID: 17220467 Free PMC article.
References
-
- Avruch J, Zhang X F, Kyriakis J M. Raf meets Ras: completing the framework of a signal transduction pathway. Trends Biochem Sci. 1994;19:279–283. - PubMed
-
- Bruder J, Heidecker G, Rapp U. Serum-, TPA-, and ras-induced expression from Ap-1/Ets-driven promoters requires Raf-1 kinase. Genes Dev. 1992;6:545–556. - PubMed
-
- Brugge J S. Interaction of the Rous sarcoma virus protein pp60src with the cellular proteins pp50 and pp90. Curr Top Microbiol Immunol. 1986;123:1–22. - PubMed
-
- Chow Y H, Pumiglia K, Jun T H, Dent P, Sturgill T W, Jove R. Functional mapping of the N-terminal regulatory domain in the human Raf-1 protein kinase. J Biol Chem. 1995;270:14100–14106. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
