Reduced phosphorylation of p50 is responsible for diminished NF-kappaB binding to the major histocompatibility complex class I enhancer in adenovirus type 12-transformed cells
- PMID: 10022903
- PMCID: PMC84009
- DOI: 10.1128/MCB.19.3.2169
Reduced phosphorylation of p50 is responsible for diminished NF-kappaB binding to the major histocompatibility complex class I enhancer in adenovirus type 12-transformed cells
Abstract
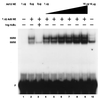
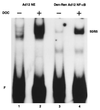
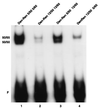
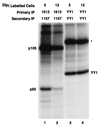
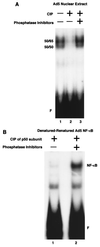
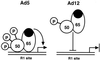
Reduced cell surface levels of major histocompatibility complex class I antigens enable adenovirus type 12 (Ad12)-transformed cells to escape immunosurveillance by cytotoxic T lymphocytes (CTL), contributing to their tumorigenic potential. In contrast, nontumorigenic Ad5-transformed cells harbor significant cell surface levels of class I antigens and are susceptible to CTL lysis. Ad12 E1A mediates down-regulation of class I transcription by increasing COUP-TF repressor binding and decreasing NF-kappaB activator binding to the class I enhancer. The mechanism underlying the decreased binding of nuclear NF-kappaB in Ad12-transformed cells was investigated. Electrophoretic mobility shift assay analysis of hybrid NF-kappaB dimers reconstituted from denatured and renatured p50 and p65 subunits from Ad12- and Ad5-transformed cell nuclear extracts demonstrated that p50, and not p65, is responsible for the decreased ability of NF-kappaB to bind to DNA in Ad12-transformed cells. Hypophosphorylation of p50 was found to correlate with restricted binding of NF-kappaB to DNA in Ad12-transformed cells. The importance of phosphorylation of p50 for NF-kappaB binding was further demonstrated by showing that an NF-kappaB dimer composed of p65 and alkaline phosphatase-treated p50 from Ad5-transformed cell nuclear extracts could not bind to DNA. These results suggest that phosphorylation of p50 is a key step in the nuclear regulation of NF-kappaB in adenovirus-transformed cells.
Figures






References
-
- Ackrill A M, Blair G E. Regulation of major histocompatibility class I gene expression at the level of transcription in highly oncogenic adenovirus transformed rat cells. Oncogene. 1988;3:483–487. - PubMed
-
- Ackrill A M, Blair G E. Nuclear proteins binding to an enhancer element of the major histocompatibility complex promoter: differences between highly oncogenic and nononcogenic adenovirus-transformed rat cells. Virology. 1989;172:643–646. - PubMed
-
- Andreu J M, de la Torre J, Carrascosa J L. Interaction of tubulin with octyl glucoside and deoxycholate. 2. Protein conformation, binding of colchicine ligands, and microtubule assembly. Biochemistry. 1986;25:5230–5239. - PubMed
-
- Andreu J M, Garcia de Ancos J, Starling D, Hodgkinson J L, Bordas J. A synchrotron X-ray scattering characterization of purified tubulin and of its expansion induced by mild detergent binding. Biochemistry. 1989;28:4036–4040. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
