A human sequence homologue of Staufen is an RNA-binding protein that is associated with polysomes and localizes to the rough endoplasmic reticulum
- PMID: 10022908
- PMCID: PMC84014
- DOI: 10.1128/MCB.19.3.2212
A human sequence homologue of Staufen is an RNA-binding protein that is associated with polysomes and localizes to the rough endoplasmic reticulum
Abstract
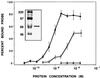
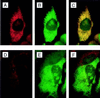
In the course of a two-hybrid screen with the NS1 protein of influenza virus, a human clone capable of coding for a protein with high homology to the Staufen protein from Drosophila melanogaster (dmStaufen) was identified. With these sequences used as a probe, cDNAs were isolated from a lambda cDNA library. The encoded protein (hStaufen-like) contained four double-stranded RNA (dsRNA)-binding domains with 55% similarity and 38% identity to those of dmStaufen, including identity at all residues involved in RNA binding. A recombinant protein containing all dsRNA-binding domains was expressed in Escherichia coli as a His-tagged polypeptide. It showed dsRNA binding activity in vitro, with an apparent Kd of 10(-9) M. Using a specific antibody, we detected in human cells a major form of the hStaufen-like protein with an apparent molecular mass of 60 to 65 kDa. The intracellular localization of hStaufen-like protein was investigated by immunofluorescence using a series of markers for the cell compartments. Colocalization was observed with the rough endoplasmic reticulum but not with endosomes, cytoskeleton, or Golgi apparatus. Furthermore, sedimentation analyses indicated that hStaufen-like protein associates with polysomes. These results are discussed in relation to the possible functions of the protein.
Figures






Similar articles
-
Interaction of influenza virus NS1 protein and the human homologue of Staufen in vivo and in vitro.Nucleic Acids Res. 1999 Jun 1;27(11):2241-7. doi: 10.1093/nar/27.11.2241. Nucleic Acids Res. 1999. PMID: 10325410 Free PMC article.
-
Mammalian staufen is a double-stranded-RNA- and tubulin-binding protein which localizes to the rough endoplasmic reticulum.Mol Cell Biol. 1999 Mar;19(3):2220-30. doi: 10.1128/MCB.19.3.2220. Mol Cell Biol. 1999. PMID: 10022909 Free PMC article.
-
The composition of Staufen-containing RNA granules from human cells indicates their role in the regulated transport and translation of messenger RNAs.Nucleic Acids Res. 2004 Apr 30;32(8):2411-20. doi: 10.1093/nar/gkh552. Print 2004. Nucleic Acids Res. 2004. PMID: 15121898 Free PMC article.
-
Compartmentation of the rough endoplasmic reticulum.Mol Cell Biochem. 1986 Jun;71(1):3-18. doi: 10.1007/BF00219323. Mol Cell Biochem. 1986. PMID: 2425244 Review.
-
Drosophila RNA binding proteins.Int Rev Cytol. 2006;248:43-139. doi: 10.1016/S0074-7696(06)48002-5. Int Rev Cytol. 2006. PMID: 16487790 Review.
Cited by
-
The double-stranded RNA-binding protein, Staufen1, is an IRES-transacting factor regulating HIV-1 cap-independent translation initiation.Nucleic Acids Res. 2022 Jan 11;50(1):411-429. doi: 10.1093/nar/gkab1188. Nucleic Acids Res. 2022. PMID: 34893869 Free PMC article.
-
Protein synthesis in the dendrite.Philos Trans R Soc Lond B Biol Sci. 2002 Apr 29;357(1420):521-9. doi: 10.1098/rstb.2001.0887. Philos Trans R Soc Lond B Biol Sci. 2002. PMID: 12028789 Free PMC article. Review.
-
Eukaryotic translation initiation factor 4GI is a cellular target for NS1 protein, a translational activator of influenza virus.Mol Cell Biol. 2000 Sep;20(17):6259-68. doi: 10.1128/MCB.20.17.6259-6268.2000. Mol Cell Biol. 2000. PMID: 10938102 Free PMC article.
-
Staufen1 is imported into the nucleolus via a bipartite nuclear localization signal and several modulatory determinants.Biochem J. 2006 Jan 1;393(Pt 1):245-54. doi: 10.1042/BJ20050694. Biochem J. 2006. PMID: 16162096 Free PMC article.
-
Protein synthesis shut-off induced by influenza virus infection is independent of PKR activity.J Virol. 2000 Sep;74(18):8781-4. doi: 10.1128/jvi.74.18.8781-8784.2000. J Virol. 2000. PMID: 10954584 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
