Mutations of oncoprotein 18/stathmin identify tubulin-directed regulatory activities distinct from tubulin association
- PMID: 10022911
- PMCID: PMC84017
- DOI: 10.1128/MCB.19.3.2242
Mutations of oncoprotein 18/stathmin identify tubulin-directed regulatory activities distinct from tubulin association
Abstract
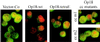
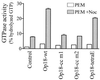
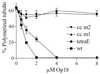
Oncoprotein 18/stathmin (Op18) is a recently identified phosphorylation-responsive regulator of the microtubule (MT) system. It was originally proposed that Op18 specifically regulates dynamic properties of MTs by associating with tubulin, but it has subsequently been proposed that Op18 acts simply by sequestering of tubulin heterodimers. We have dissected the mechanistic action of Op18 by generation of two distinct classes of mutants. One class has interruptions of the heptad repeats of a potential coiled-coil region of Op18, and the other involves substitution at all four phosphorylation sites with negatively charged Glu residues. Both types of mutation result in Op18 proteins with a limited decrease in tubulin complex formation. However, the MT-destabilizing activities of the coiled-coil mutants are more severely reduced in transfected leukemia cells than those of the Glu-substituted Op18 derivative, providing evidence for tubulin-directed regulatory activities distinct from tubulin complex formation. Analysis of Op18-mediated regulation of tubulin GTPase activity and taxol-promoted tubulin polymerization showed that while wild-type and Glu-substituted Op18 derivatives are active, the coiled-coil mutants are essentially inactive. This suggests that Op18-tubulin contact involves structural motifs that deliver a signal of regulatory importance to the MT system.
Figures

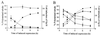
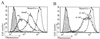
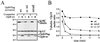
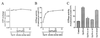
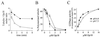
Similar articles
-
Op18/stathmin mediates multiple region-specific tubulin and microtubule-regulating activities.J Cell Biol. 1999 Sep 20;146(6):1289-302. doi: 10.1083/jcb.146.6.1289. J Cell Biol. 1999. PMID: 10491392 Free PMC article.
-
Dissociation of the tubulin-sequestering and microtubule catastrophe-promoting activities of oncoprotein 18/stathmin.Mol Biol Cell. 1999 Jan;10(1):105-18. doi: 10.1091/mbc.10.1.105. Mol Biol Cell. 1999. PMID: 9880330 Free PMC article.
-
Regulation of microtubule dynamics by extracellular signals: cAMP-dependent protein kinase switches off the activity of oncoprotein 18 in intact cells.J Cell Biol. 1998 Jan 12;140(1):131-41. doi: 10.1083/jcb.140.1.131. J Cell Biol. 1998. PMID: 9425161 Free PMC article.
-
The oncoprotein 18/stathmin family of microtubule destabilizers.Curr Opin Cell Biol. 2002 Feb;14(1):18-24. doi: 10.1016/s0955-0674(01)00289-7. Curr Opin Cell Biol. 2002. PMID: 11792540 Review.
-
Stathmin and its phosphoprotein family: general properties, biochemical and functional interaction with tubulin.Cell Struct Funct. 1999 Oct;24(5):345-57. doi: 10.1247/csf.24.345. Cell Struct Funct. 1999. PMID: 15216892 Review.
Cited by
-
The catastrophe-promoting activity of ectopic Op18/stathmin is required for disruption of mitotic spindles but not interphase microtubules.Mol Biol Cell. 2001 Jan;12(1):73-83. doi: 10.1091/mbc.12.1.73. Mol Biol Cell. 2001. PMID: 11160824 Free PMC article.
-
Op18/stathmin caps a kinked protofilament-like tubulin tetramer.EMBO J. 2000 Feb 15;19(4):572-80. doi: 10.1093/emboj/19.4.572. EMBO J. 2000. PMID: 10675326 Free PMC article.
-
Model for stathmin/OP18 binding to tubulin.EMBO J. 2000 Jan 17;19(2):213-22. doi: 10.1093/emboj/19.2.213. EMBO J. 2000. PMID: 10637225 Free PMC article.
-
Phosphorylation disrupts the central helix in Op18/stathmin and suppresses binding to tubulin.EMBO Rep. 2001 Jun;2(6):505-10. doi: 10.1093/embo-reports/kve105. EMBO Rep. 2001. PMID: 11415983 Free PMC article.
-
Op18/stathmin mediates multiple region-specific tubulin and microtubule-regulating activities.J Cell Biol. 1999 Sep 20;146(6):1289-302. doi: 10.1083/jcb.146.6.1289. J Cell Biol. 1999. PMID: 10491392 Free PMC article.
References
-
- Belmont L D, Mitchison T J. Identification of a protein that interacts with tubulin dimers and increases the catastrophe rate of microtubules. Cell. 1996;84:623–631. - PubMed
-
- Boron W F. Intracellular pH regulation in epithelial cells. Annu Rev Physiol. 1986;48:377–388. - PubMed
-
- Brattsand G, Roos G, Marklund U, Ueda H, Landberg G, Nanberg E, Sideras P, Gullberg M. Quantitative analysis of the expression and regulation of an activation-regulated phosphoprotein (oncoprotein 18) in normal and neoplastic cells. Leukemia. 1993;7:569–579. - PubMed
-
- Correia J J. Effects of antimitotic agents on tubulin-nucleotide interactions. Pharmacol Ther. 1991;52:127–147. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
