p38 mitogen-activated protein kinase can be involved in transforming growth factor beta superfamily signal transduction in Drosophila wing morphogenesis
- PMID: 10022918
- PMCID: PMC84024
- DOI: 10.1128/MCB.19.3.2322
p38 mitogen-activated protein kinase can be involved in transforming growth factor beta superfamily signal transduction in Drosophila wing morphogenesis
Abstract
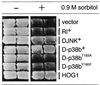
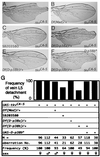
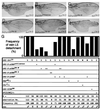
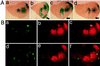
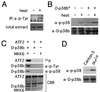
p38 mitogen-activated protein kinase (p38) has been extensively studied as a stress-responsive kinase, but its role in development remains unknown. The fruit fly, Drosophila melanogaster, has two p38 genes, D-p38a and D-p38b. To elucidate the developmental function of the Drosophila p38's, we used various genetic and pharmacological manipulations to interfere with their functions: expression of a dominant-negative form of D-p38b, expression of antisense D-p38b RNA, reduction of the D-p38 gene dosage, and treatment with the p38 inhibitor SB203580. Expression of a dominant-negative D-p38b in the wing imaginal disc caused a decapentaplegic (dpp)-like phenotype and enhanced the phenotype of a dpp mutant. Dpp is a secretory ligand belonging to the transforming growth factor beta superfamily which triggers various morphogenetic processes through interaction with the receptor Thick veins (Tkv). Inhibition of D-p38b function also caused the suppression of the wing phenotype induced by constitutively active Tkv (TkvCA). Mosaic analysis revealed that D-p38b regulates the Tkv-dependent transcription of the optomotor-blind (omb) gene in non-Dpp-producing cells, indicating that the site of D-p38b action is downstream of Tkv. Furthermore, forced expression of TkvCA induced an increase in the phosphorylated active form(s) of D-p38(s). These results demonstrate that p38, in addition to its role as a transducer of emergency stress signaling, may function to modulate Dpp signaling.
Figures


References
-
- Adachi-Yamada, T. Unpublished data.
-
- Ashburner M. Drosophila, a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989.
-
- Atfi A, Buisine M, Mazars A, Gespach C. Induction of apoptosis by DPC4, a transcriptional factor regulated by transforming growth factor-β through stress-activated protein kinase/c-Jun N-terminal kinase (SAPK/JNK) signaling pathway. J Biol Chem. 1997;272:24731–24734. - PubMed
-
- Blumer K J, Johnson G L. Diversity in function and regulation of MAP kinase pathways. Trends Biochem Sci. 1994;19:236–240. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
