Identification of a new Pyk2 target protein with Arf-GAP activity
- PMID: 10022920
- PMCID: PMC84026
- DOI: 10.1128/MCB.19.3.2338
Identification of a new Pyk2 target protein with Arf-GAP activity
Abstract
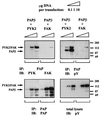
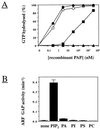
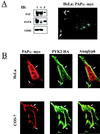
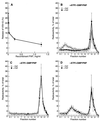
Protein tyrosine kinase Pyk2 is activated by a variety of G-protein-coupled receptors and by extracellular signals that elevate intracellular Ca2+ concentration. We have identified a new Pyk2 binding protein designated Pap. Pap is a multidomain protein composed of an N-terminal alpha-helical region with a coiled-coil motif, followed by a pleckstrin homology domain, an Arf-GAP domain, an ankyrin homology region, a proline-rich region, and a C-terminal SH3 domain. We demonstrate that Pap forms a stable complex with Pyk2 and that activation of Pyk2 leads to tyrosine phosphorylation of Pap in living cells. Immunofluorescence experiments demonstrate that Pap is localized in the Golgi apparatus and at the plasma membrane, where it is colocalized with Pyk2. In addition, in vitro recombinant Pap exhibits strong GTPase-activating protein (GAP) activity towards the small GTPases Arf1 and Arf5 and weak activity towards Arf6. Addition of recombinant Pap protein to Golgi preparations prevented Arf-dependent generation of post-Golgi vesicles in vitro. Moreover, overexpression of Pap in cultured cells reduced the constitutive secretion of a marker protein. We propose that Pap functions as a GAP for Arf and that Pyk2 may be involved in regulation of vesicular transport through its interaction with Pap.
Figures






References
-
- Astier A, Manie S N, Avraham H, Hirai H, Law S F, Zhang Y, Golemis E A, Fu Y, Druker B J, Haghayeghi N, Freedman A S, Avraham S. The related focal adhesion tyrosine kinase differentially phosphorylates p130Cas and the Cas-like protein, p105HEF1. J Biol Chem. 1997;272:19719–19724. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
