The staphylococcal transferrin-binding protein is a cell wall glyceraldehyde-3-phosphate dehydrogenase
- PMID: 10024547
- PMCID: PMC96433
- DOI: 10.1128/IAI.67.3.1086-1092.1999
The staphylococcal transferrin-binding protein is a cell wall glyceraldehyde-3-phosphate dehydrogenase
Abstract
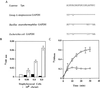
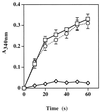
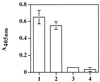
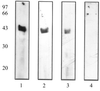
Staphylococcus aureus and Staphylococcus epidermidis possess a 42-kDa cell wall transferrin-binding protein (Tpn) which is involved in the acquisition of transferrin-bound iron. To characterize this protein further, cell wall fractions were subjected to two-dimensional sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis blotted, and the N-terminus of Tpn was sequenced. Comparison of the first 20 amino acid residues of Tpn with the protein databases revealed a high degree of homology to the glycolytic enzyme glyceraldehyde-3-phosphate dehydrogenase (GAPDH). Analysis of staphylococcal cell wall fractions for GAPDH activity confirmed the presence of a functional enzyme which, like Tpn, is regulated by the availability of iron in the growth medium. To determine whether Tpn is responsible for this GAPDH activity, it was affinity purified with NAD+ agarose. Both S. epidermidis and S. aureus Tpn catalyzed the conversion of glyceraldehyde-3-phosphate to 1,3-diphosphoglycerate. In contrast, Staphylococcus saprophyticus, which lacks a Tpn, has no cell wall-associated GAPDH activity. Native polyacrylamide gel electrophoresis of the affinity-purified Tpn revealed that it was present in the cell wall as a tetramer, consistent with the structures of all known cytoplasmic GAPDHs. Furthermore, the affinity-purified Tpn retained its ability to bind human transferrin both in its native tetrameric and SDS-denatured monomeric forms. Apart from interacting with human transferrin, Tpn, in common with the group A streptococcal cell wall GAPDH, binds human plasmin. Tpn-bound plasmin is enzymatically active and therefore may contribute to the ability of staphylococci to penetrate tissues during infections. These studies demonstrate that the staphylococcal transferrin receptor protein, Tpn, is a multifunctional cell wall GAPDH.
Figures




References
-
- Arnold H, Pette D. Bundling of glycolytic enzymes to structure proteins of the muscle. Eur J Biochem. 1968;6:163–171. - PubMed
-
- Arnold H, Pette D. Binding of aldolase and triosephosphate dehydrogenase to F-actin and modification of catalytic properties of aldolase. Eur J Biochem. 1970;15:360–366. - PubMed
-
- Cornelissen C N, Sparling P F. Iron piracy: acquisition of transferrin-bound iron by bacterial pathogens. Mol Microbiol. 1994;14:843–850. - PubMed
-
- Cornelissen C N, Anderson J E, Sparling P F. Energy-dependent changes in the gonococcal transferrin receptor. Mol Microbiol. 1997;26:25–35. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

