Transcutaneous immunization with bacterial ADP-ribosylating exotoxins as antigens and adjuvants
- PMID: 10024549
- PMCID: PMC96435
- DOI: 10.1128/IAI.67.3.1100-1106.1999
Transcutaneous immunization with bacterial ADP-ribosylating exotoxins as antigens and adjuvants
Abstract
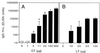
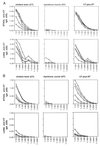
Transcutaneous immunization (TCI) is a new technique that uses the application of vaccine antigens in a solution on the skin to induce potent antibody responses without systemic or local toxicity. We have previously shown that cholera toxin (CT), a potent adjuvant for oral and nasal immunization, can induce both serum and mucosal immunoglobulin G (IgG) and IgA and protect against toxin-mediated mucosal disease when administered by the transcutaneous route. Additionally, CT acts as an adjuvant for coadministered antigens such as tetanus and diphtheria toxoids when applied to the skin. CT, a member of the bacterial ADP-ribosylating exotoxin (bARE) family, is most potent as an adjuvant when the A-B subunits are present and functional. We now show that TCI induces secondary antibody responses to coadministered antigens as well as to CT in response to boosting immunizations. IgG antibodies to coadministered antigens were also found in the stools and lung washes of immunized mice, suggesting that TCI may target mucosal pathogens. Mice immunized by the transcutaneous route with tetanus fragment C and CT developed anti-tetanus toxoid antibodies and were protected against systemic tetanus toxin challenge. We also show that bAREs, similarly organized as A-B subunits, as well as the B subunit of CT alone, induced antibody responses to themselves when given via TCI. Thus, TCI appears to induce potent, protective immune responses to both systemic and mucosal challenge and offers significant potential practical advantages for vaccine delivery.
Figures



References
-
- Brink P R G, van Loon A M, Trommelen J C M, Gribnau F W J, Smale-Novakova I R O. Virus transmission by subcutaneous jet injection. J Med Microbiol. 1985;20:393–397. - PubMed
-
- Centers for Disease Control. Hepatitis B associated with jet gun injection—California. Morbid Mortal Weekly Rep. 1986;35:373–376. - PubMed
-
- Clements J D, Jertborn M, Sack D, Stanton B, Holmgren J, Khan M R, Huda S. Effect of neutralization of gastric acid on immune responses to an oral B subunit, killed whole cell cholera vaccine. J Infect Dis. 1986;154:175–178. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

