The Pseudomonas aeruginosa secretory product pyocyanin inactivates alpha1 protease inhibitor: implications for the pathogenesis of cystic fibrosis lung disease
- PMID: 10024562
- PMCID: PMC96448
- DOI: 10.1128/IAI.67.3.1207-1212.1999
The Pseudomonas aeruginosa secretory product pyocyanin inactivates alpha1 protease inhibitor: implications for the pathogenesis of cystic fibrosis lung disease
Abstract
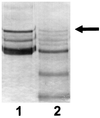
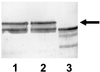
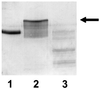
Alpha1 Protease inhibitor (alpha1PI) modulates serine protease activity in the lung. Reactive oxygen species inactivate alpha1PI, and this process has been implicated in the pathogenesis of a variety of forms of lung injury. An imbalance of protease-antiprotease activity is also detected in the airways of patients with cystic fibrosis-associated lung disease who are infected with Pseudomonas aeruginosa. P. aeruginosa secretes pyocyanin, which, through its ability to redox cycle, induces cells to generate reactive oxygen species. We tested the hypothesis that redox cycling of pyocyanin could lead to inactivation of alpha1PI. When alpha1PI was exposed to NADH and pyocyanin, a combination that results in superoxide production, alpha1PI lost its ability to form an inhibitory complex with both porcine pancreatic elastase (PPE) and trypsin. Similarly, addition of pyocyanin to cultures of human airway epithelial cells to which alpha1PI was also added resulted in a loss of the ability of alpha1PI to form a complex with PPE or trypsin. Neither superoxide dismutase, catalase, nor dimethylthiourea nor depletion of the media of O2 to prevent formation of reactive oxygen species blocked pyocyanin-mediated inactivation of alpha1PI. These data raise the possibility that a direct interaction between reduced pyocyanin and alpha1PI is involved in the process. Consistent with this possibility, pretreatment of alpha1PI with the reducing agent beta-mercaptoethanol also inhibited binding of trypsin to alpha1PI. These data suggest that pyocyanin could contribute to lung injury in the P. aeruginosa-infected airway of cystic fibrosis patients by decreasing the ability of alpha1PI to control the local activity of serine proteases.
Figures

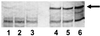


References
-
- Allen E D. Opportunities for the use of aerosolized α1-antitrypsin for the treatment of cystic fibrosis. Chest. 1996;110(Suppl.):256S–260S. - PubMed
-
- Birrer P, McElvaney N G, Rüdeberg A, Wirz Sommer C, Liechti-Gallati S, Kraemer R, Hubbard R, Crystal R G. Protease-antiprotease imbalance in the lungs of children with cystic fibrosis. Am J Respir Crit Care Med. 1994;150:207–213. - PubMed
-
- Britigan B E, Roeder T L, Rasmussen G T, Shasby D M, McCormick M L, Cox C D. Interaction of the Pseudomonas aeruginosa secretory products pyocyanin and pyochelin generates hydroxyl radical and causes synergistic damage to endothelial cells: implications for pseudomonas-associated tissue injury. J Clin Investig. 1992;90:2187–2196. - PMC - PubMed
-
- Bruce M C, Poncz L, Klinger J D, Stern R C, Tomashefski J F, Jr, Dearborn D G. Biochemical and pathologic evidence for proteolytic destruction of lung connective tissue in cystic fibrosis. Am Rev Respir Dis. 1985;132:529–535. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

