Functional activities and epitope specificity of human and murine antibodies against the class 4 outer membrane protein (Rmp) of Neisseria meningitidis
- PMID: 10024570
- PMCID: PMC96456
- DOI: 10.1128/IAI.67.3.1267-1276.1999
Functional activities and epitope specificity of human and murine antibodies against the class 4 outer membrane protein (Rmp) of Neisseria meningitidis
Abstract
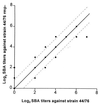
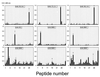
Antibodies against the class 4 outer membrane protein (OMP) from Neisseria meningitidis have been purified from sera from vaccinees immunized with the Norwegian meningococcal group B outer membrane vesicle vaccine. The human sera and purified antibodies reacted strongly with the class 4 OMP in immunoblots, whereas experiments with whole bacteria showed only weak reactions, indicating that the antibodies mainly reacted with parts of the class 4 molecule that were not exposed. The purified human anti-class 4 OMP antibodies and the monoclonal antibodies (MAbs) were neither bactericidal nor opsonic against live meningococci. Three new MAbs against the class 4 OMP were generated and compared with other, previously described MAbs. Three linear epitopes in different regions of the class 4 OMP were identified by the reaction of MAbs with synthetic peptides. The MAbs showed no blocking effect on bactericidal activity of MAbs against other OMPs. However, one of the eight purified human anti-class 4 OMP antibody preparations, selected from immunoblot reactions among sera from 27 vaccinees, inhibited at high concentrations the bactericidal effect of a MAb against the class 1 OMP. However, these antibodies were not vaccine induced, as they were present also before vaccination. Therefore, this study gave no evidence that vaccination with a meningococcal outer membrane vesicle vaccine containing the class 4 OMP induces blocking antibodies. Our data indicated that the structure of class 4 OMP does not correspond to standard beta-barrel structures of integral OMPs and that no substantial portion of the OmpA-like C-terminal region of this protein is located at the surface of the outer membrane.
Figures




References
-
- Aase A, Sandlie I, Norderhaug L, Brekke O H, Michaelsen T E. The extended hinge region of IgG3 is not required for high phagocytic capacity mediated by Fca receptors, but the heavy chains must be disulfide bonded. Eur J Immunol. 1993;23:1546–1551. - PubMed
-
- Achtman M, Kusecek B, Morelli G, Eickmann K, Wang J, Crowe B, Wall R A, Hassan-King M, Moore P S, Zollinger W D. A comparison of the variable antigens expressed by clone IV-1 and subgroup III of Neisseria meningitidis serogroup A. J Infect Dis. 1992;165:53–68. - PubMed
-
- Andersen S R, Kolberg J, Høiby E A, Namork E, Caugant D A, Frøholm L O, Jantzen E, Bjune G. Lipopolysaccharide heterogeneity and escape mechanisms of Neisseria meningitidis: possible consequences for vaccine development. Microbiol Pathol. 1997;23:139–155. - PubMed
-
- Arhin F F, Moreau F, Coulton J W, Mills E L. Subtyping of Neisseria meningitidis strains isolated in Quebec, Canada: correlation between deduced amino acid sequences and serosubtyping techniques. Can J Microbiol. 1997;43:234–238. - PubMed
-
- Arminjon F, Cadoz M, Morse S A, Rock J P, Sarafian S K. Abstracts of the Annual Meeting of the American Society for Microbiology. Washington, D.C: American Society for Microbiology; 1987. Bactericidal and opsonic activities of sera from individuals immunized with a gonococcal protein I vaccine, abstr. E-92; p. 118.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

