Role of iron in Nramp1-mediated inhibition of mycobacterial growth
- PMID: 10024586
- PMCID: PMC96472
- DOI: 10.1128/IAI.67.3.1386-1392.1999
Role of iron in Nramp1-mediated inhibition of mycobacterial growth
Abstract
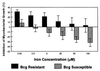
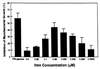
Innate resistance to mycobacterial growth is mediated by a gene, Nramp1. We have previously reported that Nramp1 mRNA from macrophages of Mycobacterium bovis BCG-resistant (Bcgr) mice is more stable than Nramp1 mRNA from macrophages of BCG-susceptible (Bcgs) mice. Based on these observations and on reports that show that the closely related Nramp2 gene is a metal ion transporter, we evaluated the effect of iron on the growth of Mycobacterium avium within macrophages as well as on the stability of Nramp1 mRNA. The addition of iron to macrophages from Bcgs mice resulted in a stimulation of mycobacterial growth. In contrast, iron increased the capacity of macrophages from Bcgr mice to control the growth of M. avium. When we treated recombinant gamma interferon (IFN-gamma)-activated macrophages with iron, we found that iron abrogated the growth inhibitory effect of IFN-gamma-activated macrophages from Bcgs mice but that it did not affect the capacity of macrophages from Bcgr mice to control microbial growth. A more detailed examination of the effect of iron on microbial growth showed that the addition of small quantities of iron to resident macrophages from Bcgr mice stimulated antimicrobial activity within a very narrow dose range. The effect of iron on the growth inhibitory activity of macrophages from Bcgr mice was abrogated by the addition of catalase or mannitol to the culture medium. These results are consistent with an Fe(II)-mediated stimulation of the Fenton/Haber-Weiss reaction and hydroxyl radical-mediated inhibition of mycobacterial growth.
Figures




Similar articles
-
Regulation of Nramp1 mRNA stability by oxidants and protein kinase C in RAW264.7 macrophages expressing Nramp1(Gly169).Biochem J. 2000 Nov 1;351 Pt 3(Pt 3):687-96. Biochem J. 2000. PMID: 11042124 Free PMC article.
-
Cytokine-mediated activation of macrophages from Mycobacterium bovis BCG-resistant and -susceptible mice: differential effects of corticosterone on antimycobacterial activity and expression of the Bcg gene (Candidate Nramp).Infect Immun. 1995 Aug;63(8):2983-8. doi: 10.1128/iai.63.8.2983-2988.1995. Infect Immun. 1995. PMID: 7622220 Free PMC article.
-
Stabilized expression of mRNA is associated with mycobacterial resistance controlled by Nramp1.Infect Immun. 1997 Feb;65(2):597-603. doi: 10.1128/iai.65.2.597-603.1997. Infect Immun. 1997. PMID: 9009318 Free PMC article.
-
The Nramp1 protein and its role in resistance to infection and macrophage function.Proc Assoc Am Physicians. 1999 Jul-Aug;111(4):283-9. doi: 10.1046/j.1525-1381.1999.99236.x. Proc Assoc Am Physicians. 1999. PMID: 10417735 Review.
-
Nramp1: a link between intracellular iron transport and innate resistance to intracellular pathogens.J Leukoc Biol. 1999 Nov;66(5):757-62. doi: 10.1002/jlb.66.5.757. J Leukoc Biol. 1999. PMID: 10577506 Review.
Cited by
-
Gallium disrupts iron metabolism of mycobacteria residing within human macrophages.Infect Immun. 2000 Oct;68(10):5619-27. doi: 10.1128/IAI.68.10.5619-5627.2000. Infect Immun. 2000. PMID: 10992462 Free PMC article.
-
Regulation of Nramp1 mRNA stability by oxidants and protein kinase C in RAW264.7 macrophages expressing Nramp1(Gly169).Biochem J. 2000 Nov 1;351 Pt 3(Pt 3):687-96. Biochem J. 2000. PMID: 11042124 Free PMC article.
-
Eat Prey, Live: Dictyostelium discoideum As a Model for Cell-Autonomous Defenses.Front Immunol. 2018 Jan 4;8:1906. doi: 10.3389/fimmu.2017.01906. eCollection 2017. Front Immunol. 2018. PMID: 29354124 Free PMC article. Review.
-
A Leishmania amazonensis ZIP family iron transporter is essential for parasite replication within macrophage phagolysosomes.J Exp Med. 2006 Oct 2;203(10):2363-75. doi: 10.1084/jem.20060559. Epub 2006 Sep 25. J Exp Med. 2006. PMID: 17000865 Free PMC article.
-
Sex- and age-dependent association of SLC11A1 polymorphisms with tuberculosis in Chinese: a case control study.BMC Infect Dis. 2007 Mar 19;7:19. doi: 10.1186/1471-2334-7-19. BMC Infect Dis. 2007. PMID: 17371589 Free PMC article.
References
-
- Alcantara O, Obeid L, Hannun Y, Ponka P, Boldt D H. Regulation of protein kinase C (PKC) expression by iron: PKC-beta and PKC-alpha gene expression and role of the 5′ flanking region of the PKC beta gene in the response to ferric transferrin. Blood. 1994;84:3510–3517. - PubMed
-
- Atkinson P G P, Barton C H. Ectopic expression of Nramp1 in COS-1 cells modulates iron accumulation. FEBS Lett. 1998;425:239–242. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

