Safety and immunogenicity of a Pseudomonas aeruginosa hybrid outer membrane protein F-I vaccine in human volunteers
- PMID: 10024596
- PMCID: PMC96482
- DOI: 10.1128/IAI.67.3.1461-1470.1999
Safety and immunogenicity of a Pseudomonas aeruginosa hybrid outer membrane protein F-I vaccine in human volunteers
Abstract
A hybrid protein [Met-Ala-(His)6OprF190-342-OprI21-83] consisting of the mature outer membrane protein I (OprI) and amino acids 190 to 342 of OprF of Pseudomonas aeruginosa was expressed in Escherichia coli and purified by Ni2+ chelate-affinity chromatography. After safety and pyrogenicity evaluations in animals, four groups of eight adult human volunteers were vaccinated intramuscularly three times at 4-week intervals and revaccinated 6 months later with either 500, 100, 50, or 20 microg of OprF-OprI adsorbed onto A1(OH)3. All vaccinations were well tolerated. After the first vaccination, a significant rise of antibody titers against P. aeruginosa OprF and OprI was measured in volunteers receiving the 100- or the 500-microg dose. After the second vaccination, significant antibody titers were measured for all groups. Elevated antibody titers against OprF and OprI could still be measured 6 months after the third vaccination. The capacity of the elicited antibodies to promote complement binding and opsonization could be demonstrated by a C1q-binding assay and by the in vitro opsonophagocytic uptake of P. aeruginosa bacteria. These data support the continued development of an OprF-OprI vaccine for use in humans.
Figures
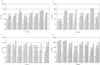
 , day 0;
, day 0;  , day 14;
, day 14;  , day 42;
, day 42;  , day 70;
, day 70;  , day 240;
, day 240;  , day 254.
, day 254.
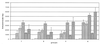
 , day 42;
, day 42;  , day 70;
, day 70;  , day 240;
, day 240;  , day 254.
, day 254.
 the third vaccination. Plates were coated with OprF-OprI and incubated with 50 μl of the respective 1:4-diluted serum and 50 μl of complement source. Binding was measured with peroxidase-linked anti-C1q antibodies, and ortho-phenylen-diamine was used as the substrate.
the third vaccination. Plates were coated with OprF-OprI and incubated with 50 μl of the respective 1:4-diluted serum and 50 μl of complement source. Binding was measured with peroxidase-linked anti-C1q antibodies, and ortho-phenylen-diamine was used as the substrate.References
-
- Alexander J W, Fisher M W. Immunization against Pseudomonas infection after thermal injury. J Infect Dis. 1974;130:152–158. - PubMed
-
- Ausubel F M, Brent R, Kingston R E, Moore D D, Smith J A, Seidman J G, Struhl K, editors. Current protocols in molecular biology 1994–1997. New York, N.Y: John Wiley and Sons; 1997. High efficiency transformation by electroporation; pp. 1.8.4–1.8.6.
-
- Benerjee S S, Emori T G, Culver D H, Gaynes R P, Jarvis W R, Horan T, Edwards J R, Henderson T, Martone W J. Secular trends in nosocomial primary bloodstream infections in the United States, 1980–1989. Am J Med. 1991;91:86S–89S. - PubMed
-
- Cohen J. Naked DNA points way to vaccines. Science. 1993;259:1691–1692. - PubMed
-
- Cryz S J, Jr, Sadoff J C, Fürer E. Octavalent Pseudomonas aeruginosa-O-polysaccharide-toxin A conjugate vaccine. Microb Pathog. 1987;6:75–80. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

