Activation of G12/G13 results in shape change and Rho/Rho-kinase-mediated myosin light chain phosphorylation in mouse platelets
- PMID: 10037795
- PMCID: PMC2132941
- DOI: 10.1083/jcb.144.4.745
Activation of G12/G13 results in shape change and Rho/Rho-kinase-mediated myosin light chain phosphorylation in mouse platelets
Abstract
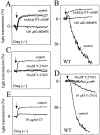
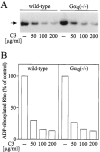
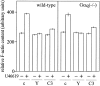
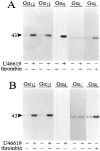
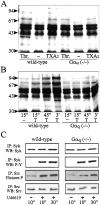
Platelets respond to various stimuli with rapid changes in shape followed by aggregation and secretion of their granule contents. Platelets lacking the alpha-subunit of the heterotrimeric G protein Gq do not aggregate and degranulate but still undergo shape change after activation through thromboxane-A2 (TXA2) or thrombin receptors. In contrast to thrombin, the TXA2 mimetic U46619 led to the selective activation of G12 and G13 in Galphaq-deficient platelets indicating that these G proteins mediate TXA2 receptor-induced shape change. TXA2 receptor-mediated activation of G12/G13 resulted in tyrosine phosphorylation of pp72(syk) and stimulation of pp60(c-src) as well as in phosphorylation of myosin light chain (MLC) in Galphaq-deficient platelets. Both MLC phosphorylation and shape change induced through G12/G13 in the absence of Galphaq were inhibited by the C3 exoenzyme from Clostridium botulinum, by the Rho-kinase inhibitor Y-27632 and by cAMP-analogue Sp-5,6-DCl-cBIMPS. These data indicate that G12/G13 couple receptors to tyrosine kinases as well as to the Rho/Rho-kinase-mediated regulation of MLC phosphorylation. We provide evidence that G12/G13-mediated Rho/Rho-kinase-dependent regulation of MLC phosphorylation participates in receptor-induced platelet shape change.
Figures


References
-
- Aktories K, Jakobs KH. Ni-mediated inhibition of human platelet adenylate cyclase by thrombin. Eur J Biochem. 1984;145:333–338. - PubMed
-
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J Biol Chem. 1996;271:20246–20249. - PubMed
-
- Amano M, Chihara K, Nakamura N, Fukata Y, Yano T, Shibata M, Ikebe M, Kaibuchi K. Myosin II activation promotes neurite retraction during the action of Rho and Rho-kinase. Genes Cells. 1998;3:177–188. - PubMed
-
- Barrett K, Leptin M, Settleman J. The Rho GTPase and a putative RhoGEF mediate a signaling pathway for the cell shape changes in Drosophilagastrulation. Cell. 1997;91:905–915. - PubMed
-
- Brass LF, Woolkalis MJ, Manning DR. Interactions in platelets between G proteins and the agonists that stimulate phospholipase C and inhibit adenylyl cyclase. J Biol Chem. 1988;263:5348–5355. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

