Comparative study of the anti-human cytomegalovirus activities and toxicities of a tetrahydrofuran phosphonate analogue of guanosine and cidofovir
- PMID: 10049267
- PMCID: PMC89160
- DOI: 10.1128/AAC.43.3.557
Comparative study of the anti-human cytomegalovirus activities and toxicities of a tetrahydrofuran phosphonate analogue of guanosine and cidofovir
Abstract
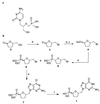
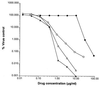
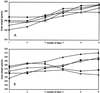
Cidofovir is the first nucleoside monophosphate analogue currently being used for the treatment of human cytomegalovirus (HCMV) retinitis in individuals with AIDS. Unfortunately, the period of therapy with the use of this compound may be limited due to the possible emergence of serious irreversible nephrotoxic effects. New drugs with improved toxicity profiles are needed. The goal of this study was to investigate the anticytomegaloviral properties and drug-induced toxicity of a novel phosphonate analogue, namely, (-)-2-(R)-dihydroxyphosphinoyl-5-(S)-(guanin-9'-yl-methyl) tetrahydrofuran (compound 1), in comparison with those of cidofovir. The inhibitory activities of both compounds on HCMV propagation in vitro were similar against the AD 169 and Towne strains, with 50% inhibitory concentrations ranging from 0.02 to 0.17 microgram/ml for cidofovir and < 0.05 to 0.09 microgram/ml for compound 1. A clinical HCMV isolate that was resistant to ganciclovir and that had a known mutation within the UL54 DNA polymerase gene and a cidofovir-resistant laboratory strain derived from strain AD 169 remained sensitive to compound 1, whereas their susceptibilities to ganciclovir and cidofovir were reduced by 33- and 10-fold, respectively. Both compound 1 and cidofovir exhibited equal potencies in an experimentally induced murine cytomegalovirus (MCMV) infection in mice, with a prevention or prolongation of mean day to death at dosages of 1.0, 3.2, and 10.0 mg/kg of body weight/day. In cytotoxicity experiments, compound 1 was found to be generally more toxic than cidofovir in cell lines Hs68, HFF, and 3T3-L1 (which are permissive for HCMV or MCMV replication) but less toxic than cidofovir in MRC-5 cells (which are permissive for HCMV replication). Drug-induced toxic side effects were noticed for both compounds in rats and guinea pigs in a 5-day repeated-dose study. In guinea pigs, a greater weight loss was noticed with cidofovir than with compound 1 at dosages of 3.0 and 10.0 mg/kg/day. An opposite effect was detected in rats, which were treated with the compounds at relatively high dosages (up to 100 mg/kg/day). Compound 1 and cidofovir were nephrotoxic in both rats and guinea pigs, with the epithelium lining the proximal convoluted tubules in the renal cortex being the primary target site. The incidence and the severity of the lesions were found to be dose dependent. The lesions observed were characterized by cytoplasm degeneration and nuclear modifications such as karyomegaly, the presence of pseudoinclusions, apoptosis, and degenerative changes. In the guinea pig model, a greater incidence and severity of lesions were observed for cidofovir than for compound 1 (P < 0.001) with a drug regimen of 10 mg/kg/day.
Figures



References
-
- Aduma P, Connelly M C, Srinivas R V, Fridland A. Metabolic diversity and antiviral activities of acyclic nucleoside phosphonates. Mol Pharmacol. 1995;47:816–822. - PubMed
-
- Baldanti F, Underwood M R, Stanat S C, Biron K K, Chou S, Sarasini A, Silini E, Gerna G. Single amino acid changes in the DNA polymerase confer foscarnet resistance and slow-growth phenotype, while mutations in the UL97-encoded phosphotransferase confer ganciclovir resistance in three double-resistant human cytomegalovirus strains recovered from patients with AIDS. J Virol. 1996;70:1390–1395. - PMC - PubMed
-
- Barnard J A, Huffman J H, Sidwell R W, Reist E J. Selective inhibition of cytomegalovirus by 9-(3′-ethylphosphono-1′-propyloxy-methyl)guanine. Antivir Res. 1993;22:77–89. - PubMed
-
- Biron K K. Ganciclovir-resistant human cytomegalovirus clinical isolates: resistance mechanisms and in vitro susceptibility to antiviral agents. Transplant Proc. 1991;23:162–167. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

