Safety and single-dose pharmacokinetics of abacavir (1592U89) in human immunodeficiency virus type 1-infected children
- PMID: 10049275
- PMCID: PMC89168
- DOI: 10.1128/AAC.43.3.609
Safety and single-dose pharmacokinetics of abacavir (1592U89) in human immunodeficiency virus type 1-infected children
Abstract
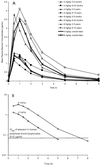
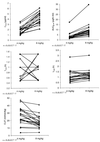
Abacavir (formerly 1592U89) is a potent 2'-deoxyguanosine analog reverse transcriptase inhibitor that has been demonstrated to have a favorable safety profile in initial clinical trials with adults with human immunodeficiency virus (HIV) type 1 infection. A phase I study was conducted to evaluate the pharmacokinetics and safety of abacavir following the administration of two single oral doses (4 and 8 mg/kg of body weight) to 22 HIV-infected children ages 3 months to 13 years. Plasma was collected for analysis at predose and at 0.5, 1, 1.5, 2, 2.5, 3, 5, and 8 h after the administration of each dose. Plasma abacavir concentrations were determined by high-performance liquid chromatography, and data were analyzed by noncompartmental methods. Abacavir was well tolerated by all subjects. The single abacavir-related adverse event was rash, which occurred in 2 of 22 subjects. After administration of the oral solution, abacavir was rapidly absorbed, with the time to the peak concentration in plasma occurring within 1.5 h postdosing. Pharmacokinetic parameter estimates were comparable among the different age groups for each dose level. The mean maximum concentration in plasma (Cmax) and the mean area under the curve from time zero to infinity (AUC0-infinity) increased by 16 and 45% more than predicted, respectively, as the abacavir dose was doubled from 4 to 8 mg/kg (Cmax increased from 1.69 to 3.94 micrograms/ml, and AUC0-infinity increased from 2.82 to 8.09 micrograms.h/ml). Abacavir was rapidly eliminated, with a mean elimination half-life of 0.98 to 1.13 h. The mean apparent clearance from plasma decreased from 27.35 to 18.88 ml/min/kg as the dose increased. Neither body surface area nor creatinine clearance were correlated with pharmacokinetic estimates at either dose. The extent of exposure to abacavir appears to be slightly lower in children than in adults, with the comparable unit doses being based on body weight. In conclusion, this study showed that abacavir is safe and well tolerated in children when it is administered as a single oral dose of 4 or 8 mg/kg.
Figures
References
-
- Balis F M, Pizzo P A, Murphy R F, Eddy J, Jarosinski P F, Falloon J, Broder S, Poplack D G. The pharmacokinetics of zidovudine administered by continuous infusion in children. Ann Intern Med. 1989;110:279–285. - PubMed
-
- Balis F M, Pizzo P A, Eddy J, Wilfert C, McKinney R, Scott G, Murphy R F, Jaroninski P F, Falloon J, Poplack D G. Pharmacokinetics of zidovudine administered intravenously and orally in children with human immunodeficiency virus infection. J Pediatr. 1989;114:880–884. - PubMed
-
- Balis F M, Pizzo P A, Butler K M, Hawkins M E, Brouwers P, Husson R N, Jacobsen F, Blaney S M, Gress J, Jaronski P, Poplack D G. Clinical pharmacology of 2′,3′-dideoxyinosine in human immunodeficiency virus-infected children. J Infect Dis. 1992;165:99–104. - PubMed
-
- Boucher F D, Modlin J F, Weller S, Ruff A, Mirochnick M, Pelton S, Wilfert C, McKinney R, Crain M J, Elkins M M, Blum M R, Prober C G. Phase I evaluation of zidovudine administered to infants exposed at birth to the human immunodeficiency virus. J Pediatr. 1993;122:137–144. - PubMed
-
- Centers for Disease Control and Prevention. Classification system for HIV infection in children under 13 years of age. Official authorized addenda: human immunodeficiency virus infection codes and official guidelines for coding and reporting ICD-9-M. Morbid Mortal Weekly Rep. 1994;43(No. RR-12):1–19.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

