Bmp4 is required for the generation of primordial germ cells in the mouse embryo
- PMID: 10049358
- PMCID: PMC316469
- DOI: 10.1101/gad.13.4.424
Bmp4 is required for the generation of primordial germ cells in the mouse embryo
Abstract
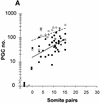
In many organisms the allocation of primordial germ cells (PGCs) is determined by the inheritance of maternal factors deposited in the egg. However, in mammals, inductive cell interactions are required around gastrulation to establish the germ line. Here, we show that Bmp4 homozygous null embryos contain no PGCs. They also lack an allantois, an extraembryonic mesodermal tissue derived, like the PGCs, from precursors in the proximal epiblast. Heterozygotes have fewer PGCs than normal, due to a reduction in the size of the founding population and not to an effect on its subsequent expansion. Analysis of beta-galactosidase activity in Bmp4(lacZneo) embryos reveals that prior to gastrulation, Bmp4 is expressed in the extraembryonic ectoderm. Later, Bmp4 is expressed in the extraembryonic mesoderm, but not in PGCs. Chimera analysis indicates that it is the Bmp4 expression in the extraembryonic ectoderm that regulates the formation of allantois and primordial germ cell precursors, and the size of the founding population of PGCs. The initiation of the germ line in the mouse therefore depends on a secreted signal from the previously segregated, extraembryonic, trophectoderm lineage.
Figures
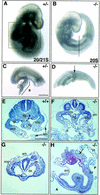
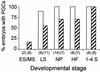



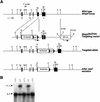

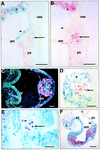

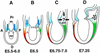
Comment on
-
Signaling for germ cells.Genes Dev. 1999 Feb 15;13(4):373-6. doi: 10.1101/gad.13.4.373. Genes Dev. 1999. PMID: 10049351 No abstract available.
References
-
- Albano RM, Arkell R, Beddington RSP, Smith JC. Expression of inhibin subunits and follistatin during postimplantation mouse development: Decidual expression of activin and expression of follistatin in primitive streak, somites and hindbrain. Development. 1994;120:803–813. - PubMed
-
- Beddington RSP, Robertson EJ. An assessment of the developmental potential of embryonic stem cells in the midgestation mouse embryo. Development. 1989;105:733–737. - PubMed
-
- ————— Anterior patterning in the mouse. Trends Genet. 1998;14:277–284. - PubMed
-
- Belo JA, Bouwmeester T, Leyns L, Kertesz N, Gallo M, Follettie M, De Robertis EM. Cerberus-like is a secreted factor with neuralizing activity expressed in the anterior primitive endoderm of the mouse gastrula. Mech Dev. 1997;68:45–57. - PubMed
-
- Biben C, Stanley E, Fabri L, Kotecha S, Rhinn M, Drinkwater C, Lah M, Wang CC, Nash A, Hilton D, Ang SL, Mohun T, Harvey RP. Murine cerberus homologue mCer-1: A candidate anterior patterning molecule. Dev Biol. 1998;194:135–151. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
