ELAV tumor antigen, Hel-N1, increases translation of neurofilament M mRNA and induces formation of neurites in human teratocarcinoma cells
- PMID: 10049360
- PMCID: PMC316478
- DOI: 10.1101/gad.13.4.449
ELAV tumor antigen, Hel-N1, increases translation of neurofilament M mRNA and induces formation of neurites in human teratocarcinoma cells
Abstract
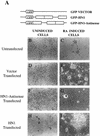
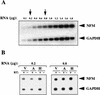
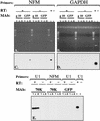
Human ELAV proteins are implicated in cell growth and differentiation via regulation of mRNA expression in the cytoplasm. In human embryonic teratocarcinoma (hNT2) cells transfected with the human neuronal ELAV-like protein, Hel-N1, neurites formed, yet cells were not terminally differentiated. Cells in which neurite formation was associated with Hel-N1 overexpression, also expressed increased levels of endogenous neurofilament M (NF-M) protein, which distributed along the neurites. However, steady-state levels of NF-M mRNA remained similar whether or not hNT2 cells were transfected with Hel-N1. These findings suggest that turnover of NF-M mRNA was not affected by Hel-N1 expression, despite the fact that Hel-N1 can bind to the 3' UTR of NF-M mRNA and was found directly associated with NF-M mRNA in transfected cells. Analysis of the association of NF-M mRNA with the translational apparatus in Hel-N1 transfectants showed nearly complete recruitment to heavy polysomes, indicating that Hel-N1 caused an increase in translational initiation. Our results suggest that the stability and/or translation of ARE-containing mRNAs can be regulated independently by the ELAV protein, Hel-N1, depending upon sequence elements in the 3' UTRs and upon the inherent turnover rates of the mRNAs that are bound to Hel-N1 in vivo.
Figures




References
-
- Andrews PW. Retinoic acid induces neuronal differentiation of a cloned human embryonal carcinoma cell line in vitro. Dev Biol. 1984;103:285–293. - PubMed
-
- Antic D, Keene JD. Messenger ribonucleoprotein complexes containing human ELAV proteins: Interactions with cytoskeleton and translational apparatus. J Cell Sci. 1998;111:183–197. - PubMed
-
- Atasoy U, Watson J, Keene JD. Ubiquitously expressed ELAV RNA-binding protein, HuA, localizes to both the nucleus and cytoplasm and is upregulated during serum stimulation and T cell activation. J Cell Sci. 1998;111:3145–3156. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
