The thioredoxin superfamily: redundancy, specificity, and gray-area genomics
- PMID: 10049365
- PMCID: PMC93523
- DOI: 10.1128/JB.181.5.1375-1379.1999
The thioredoxin superfamily: redundancy, specificity, and gray-area genomics
Figures
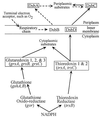
References
-
- Andersen C L, Matthey-Dupraz A, Missiakas D, Raina S. A new Escherichia coli gene, dsbG, encodes a periplasmic protein involved in disulphide bond formation, required for recycling DsbA/DsbB and DsbC redox proteins. Mol Microbiol. 1997;26:121–132. - PubMed
-
- Åslund F, Berndt K D, Holmgren A. Redox potentials of glutaredoxins and other thiol-disulfide oxidoreductases of the thioredoxin superfamily determined by direct protein-protein redox equilibria. J Biol Chem. 1997;272:30780–30786. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

