Mutational analysis of Bacillus subtilis glutamine phosphoribosylpyrophosphate amidotransferase propeptide processing
- PMID: 10049369
- PMCID: PMC93527
- DOI: 10.1128/JB.181.5.1403-1408.1999
Mutational analysis of Bacillus subtilis glutamine phosphoribosylpyrophosphate amidotransferase propeptide processing
Abstract
Glutamine phosphoribosylpyrophosphate amidotransferase from Bacillus subtilis is a member of an N-terminal nucleophile hydrolase enzyme superfamily, several of which undergo autocatalytic propeptide processing to generate the mature active enzyme. A series of mutations was analyzed to determine whether amino acid residues required for catalysis are also used for propeptide processing. Propeptide cleavage was strongly inhibited by replacement of the cysteine nucleophile and two residues of an oxyanion hole that are required for glutaminase function. However, significant propeptide processing was retained in a deletion mutant with multiple defects in catalysis that was devoid of enzyme activity. Intermolecular processing of noncleaved mutant enzyme subunits by active wild-type enzyme subunits was not detected in hetero-oligomers obtained from a coexpression experiment. While direct in vitro evidence for autocatalytic propeptide cleavage was not obtained, the results indicate that some but not all of the amino acid residues that have a role in catalysis are also needed for propeptide processing.
Figures

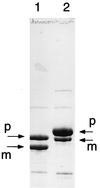
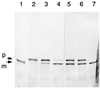
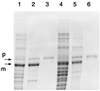
Similar articles
-
Mutational analysis of the glutamine phosphoribosylpyrophosphate amidotransferase pro-peptide.J Biol Chem. 1988 Mar 5;263(7):3323-7. J Biol Chem. 1988. PMID: 3125178
-
Avian glutamine phosphoribosylpyrophosphate amidotransferase propeptide processing and activity are dependent upon essential cysteine residues.J Biol Chem. 1992 Apr 15;267(11):7936-42. J Biol Chem. 1992. PMID: 1560022
-
Cloning and expression of avian glutamine phosphoribosylpyrophosphate amidotransferase. Conservation of a bacterial propeptide sequence supports a role for posttranslational processing.J Biol Chem. 1990 Dec 5;265(34):21152-9. J Biol Chem. 1990. PMID: 2123487
-
Role of NifS in maturation of glutamine phosphoribosylpyrophosphate amidotransferase.J Bacteriol. 1997 Dec;179(23):7587-90. doi: 10.1128/jb.179.23.7587-7590.1997. J Bacteriol. 1997. PMID: 9393728 Free PMC article.
-
Glutamine amidotransferase function. Replacement of the active-site cysteine in glutamine phosphoribosylpyrophosphate amidotransferase by site-directed mutagenesis.J Biol Chem. 1984 Nov 25;259(22):14230-6. J Biol Chem. 1984. PMID: 6094545
Cited by
-
Autoproteolytic activation of human aspartylglucosaminidase.Biochem J. 2004 Mar 1;378(Pt 2):363-71. doi: 10.1042/BJ20031496. Biochem J. 2004. PMID: 14616088 Free PMC article.
References
-
- Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. - PubMed
-
- Brannigan J A, Dodson G, Duggleby H J, Moody P C E, Smith J L, Tomchick D R, Murzin A G. A protein catalytic framework with an N-terminal nucleophile is capable of self-activation. Nature. 1995;378:416–419. - PubMed
-
- Chen P, Hochstrasser M. Autocatalytic subunit processing couples active site formation in the 20S proteasome to completion of assembly. Cell. 1996;86:961–972. - PubMed
-
- Chen S, Tomchick D R, Wolle D, Hua P, Smith J L, Switzer R L, Zalkin H. Mechanism of the synergistic end-product regulation of Bacillus subtilis glutamine phosphoribosylpyrophosphate amidotransferase by nucleotides. Biochemistry. 1997;36:10718–10726. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

