Ferritin mutants of Escherichia coli are iron deficient and growth impaired, and fur mutants are iron deficient
- PMID: 10049371
- PMCID: PMC93529
- DOI: 10.1128/JB.181.5.1415-1428.1999
Ferritin mutants of Escherichia coli are iron deficient and growth impaired, and fur mutants are iron deficient
Abstract
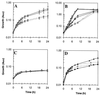
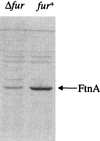
Escherichia coli contains at least two iron storage proteins, a ferritin (FtnA) and a bacterioferritin (Bfr). To investigate their specific functions, the corresponding genes (ftnA and bfr) were inactivated by replacing the chromosomal ftnA and bfr genes with disrupted derivatives containing antibiotic resistance cassettes in place of internal segments of the corresponding coding regions. Single mutants (ftnA::spc and bfr::kan) and a double mutant (ftnA::spc bfr::kan) were generated and confirmed by Western and Southern blot analyses. The iron contents of the parental strain (W3110) and the bfr mutant increased by 1.5- to 2-fold during the transition from logarithmic to stationary phase in iron-rich media, whereas the iron contents of the ftnA and ftnA bfr mutants remained unchanged. The ftnA and ftnA bfr mutants were growth impaired in iron-deficient media, but this was apparent only after the mutant and parental strains had been precultured in iron-rich media. Surprisingly, ferric iron uptake regulation (fur) mutants also had very low iron contents (2.5-fold less iron than Fur+ strains) despite constitutive expression of the iron acquisition systems. The iron deficiencies of the ftnA and fur mutants were confirmed by Mössbauer spectroscopy, which further showed that the low iron contents of ftnA mutants are due to a lack of magnetically ordered ferric iron clusters likely to correspond to FtnA iron cores. In combination with the fur mutation, ftnA and bfr mutations produced an enhanced sensitivity to hydroperoxides, presumably due to an increase in production of "reactive ferrous iron." It is concluded that FtnA acts as an iron store accommodating up to 50% of the cellular iron during postexponential growth in iron-rich media and providing a source of iron that partially compensates for iron deficiency during iron-restricted growth. In addition to repressing the iron acquisition systems, Fur appears to regulate the demand for iron, probably by controlling the expression of iron-containing proteins. The role of Bfr remains unclear.
Figures




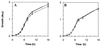
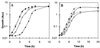

References
-
- Andrews S C. Iron storage in bacteria. Adv Microb Physiol. 1998;40:281–351. - PubMed
-
- Andrews S C, Le Brun N E, Barynin V, Thomson A J, Moore G R, Guest J R, Harrison P M. Site-directed replacement of the coaxial heme ligands of bacterioferritin generates heme-free variants. J Biol Chem. 1995;270:23268–23274. - PubMed
-
- Andrews S C, Smith J M A, Hawkins C, Williams J M, Harrison P M, Guest J R. Overproduction, purification and characterization of the bacterioferritin of Escherichia coli and a C-terminally extended variant. Eur J Biochem. 1993;213:329–338. - PubMed
-
- Bagg A, Neilands J B. Ferric uptake regulation protein acts as a repressor, employing iron(II) as co-factor to bind the operator of an iron transport operon in Escherichia coli. Biochemistry. 1987;26:5471–5477. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

