Redundant systems of phosphatidic acid biosynthesis via acylation of glycerol-3-phosphate or dihydroxyacetone phosphate in the yeast Saccharomyces cerevisiae
- PMID: 10049376
- PMCID: PMC93534
- DOI: 10.1128/JB.181.5.1458-1463.1999
Redundant systems of phosphatidic acid biosynthesis via acylation of glycerol-3-phosphate or dihydroxyacetone phosphate in the yeast Saccharomyces cerevisiae
Abstract
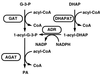
In the yeast Saccharomyces cerevisiae lipid particles harbor two acyltransferases, Gat1p and Slc1p, which catalyze subsequent steps of acylation required for the formation of phosphatidic acid. Both enzymes are also components of the endoplasmic reticulum, but this compartment contains additional acyltransferase(s) involved in the biosynthesis of phosphatidic acid (K. Athenstaedt and G. Daum, J. Bacteriol. 179:7611-7616, 1997). Using the gat1 mutant strain TTA1, we show here that Gat1p present in both subcellular fractions accepts glycerol-3-phosphate and dihydroxyacetone phosphate as a substrate. Similarly, the additional acyltransferase(s) present in the endoplasmic reticulum can acylate both precursors. In contrast, yeast mitochondria harbor an enzyme(s) that significantly prefers dihydroxyacetone phosphate as a substrate for acylation, suggesting that at least one additional independent acyltransferase is present in this organelle. Surprisingly, enzymatic activity of 1-acyldihydroxyacetone phosphate reductase, which is required for the conversion of 1-acyldihydroxyacetone phosphate to 1-acylglycerol-3-phosphate (lysophosphatidic acid), is detectable only in lipid particles and the endoplasmic reticulum and not in mitochondria. In vivo labeling of wild-type cells with [2-3H, U-14C]glycerol revealed that both glycerol-3-phosphate and dihydroxyacetone phosphate can be incorporated as a backbone of glycerolipids. In the gat1 mutant and the 1-acylglycerol-3-phosphate acyltransferase slc1 mutant, the dihydroxyacetone phosphate pathway of phosphatidic acid biosynthesis is slightly preferred as compared to the wild type. Thus, mutations of the major acyltransferases Gat1p and Slc1p lead to an increased contribution of mitochondrial acyltransferase(s) to glycerolipid synthesis due to their substrate preference for dihydroxyacetone phosphate.
Figures


References
-
- Bell R M, Coleman R A. Enzymes of triacylglycerol formation in mammals. In: Boyer P D, editor. The enzymes. New York, N.Y: Academic Press; 1983. pp. 87–111.
-
- Christiansen K. Triacylglycerol synthesis in lipid particles from baker’s yeast (Saccharomyces cerevisiae) Biochim Biophys Acta. 1978;530:78–90. - PubMed
-
- Daum G, Boehni P C, Schatz G. Import of proteins into mitochondria. Cytochrome b2 and cytochrome c peroxidase are located in the intermembrane space of yeast mitochondria. J Biol Chem. 1982;257:13028–13033. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

