Induction of defense responses in cucumber plants (Cucumis sativus L. ) By the biocontrol agent trichoderma harzianum
- PMID: 10049864
- PMCID: PMC91145
- DOI: 10.1128/AEM.65.3.1061-1070.1999
Induction of defense responses in cucumber plants (Cucumis sativus L. ) By the biocontrol agent trichoderma harzianum
Abstract
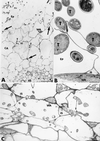
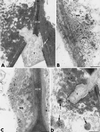
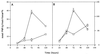
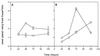
The potential of the biocontrol agent Trichoderma harzianum T-203 to trigger plant defense responses was investigated by inoculating roots of cucumber seedlings with Trichoderma in an aseptic, hydroponic system. Trichoderma-treated plants were more developed than nontreated plants throughout the experiment. Electron microscopy of ultrathin sections from Trichoderma-treated roots revealed penetration of Trichoderma into the roots, restricted mainly to the epidermis and outer cortex. Strengthening of the epidermal and cortical cell walls was observed, as was the deposition of newly formed barriers. These typical host reactions were found beyond the sites of potential fungal penetration. Wall appositions contained large amounts of callose and infiltrations of cellulose. The wall-bound chitin in Trichoderma hyphae was preserved, even when the hyphae had undergone substantial disorganization. Biochemical analyses revealed that inoculation with Trichoderma initiated increased peroxidase and chitinase activities within 48 and 72 h, respectively. These results were observed for both the roots and the leaves of treated seedlings, providing evidence that T. harzianum may induce systemic resistance mechanisms in cucumber plants.
Figures


References
-
- Baker R. Improved Trichoderma spp. for promoting crop productivity. Trends Biotech. 1989;7:34–38.
-
- Benhamou N. Immunocytochemistry of plant defense mechanisms induced upon microbial attack. Microsc Res Tech. 1995;31:63–78. - PubMed
-
- Benhamou N. Preparation and application of lectin-gold complexes. In: Hayat M A, editor. Colloidal gold, principles, methods, and applications. Vol. 1. New York, N.Y: Academic Press, Inc.; 1989. pp. 95–143.
-
- Benhamou N. Ultrastructural detection of β-1,3-glucans in tobacco root tissues infected by Phytophthora parasitica var. nicotianae using a gold-complexed tobacco β-1,3-glucanase. Physiol Mol Plant Pathol. 1992;41:351–370.
-
- Benhamou N, Chamberland H, Ouellette G B, Pauzé F J. Ultrastructural localization of β-1,4-d-glucans in two pathogenic fungi and in their host tissues by means of an exoglucanase-gold complex. Can J Microbiol. 1987;33:405–417.
LinkOut - more resources
Full Text Sources
Other Literature Sources

