Contrasting effects of a nonionic surfactant on the biotransformation of polycyclic aromatic hydrocarbons to cis-dihydrodiols by soil bacteria
- PMID: 10049904
- PMCID: PMC91185
- DOI: 10.1128/AEM.65.3.1335-1339.1999
Contrasting effects of a nonionic surfactant on the biotransformation of polycyclic aromatic hydrocarbons to cis-dihydrodiols by soil bacteria
Abstract
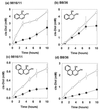
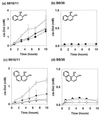
The biotransformation of the polycyclic aromatic hydrocarbons (PAHs) naphthalene and phenanthrene was investigated by using two dioxygenase-expressing bacteria, Pseudomonas sp. strain 9816/11 and Sphingomonas yanoikuyae B8/36, under conditions which facilitate mass-transfer limited substrate oxidation. Both of these strains are mutants that accumulate cis-dihydrodiol metabolites under the reaction conditions used. The effects of the nonpolar solvent 2,2,4, 4,6,8,8-heptamethylnonane (HMN) and the nonionic surfactant Triton X-100 on the rate of accumulation of these metabolites were determined. HMN increased the rate of accumulation of metabolites for both microorganisms, with both substrates. The enhancement effect was most noticeable with phenanthrene, which has a lower aqueous solubility than naphthalene. Triton X-100 increased the rate of oxidation of the PAHs with strain 9816/11 with the effect being most noticeable when phenanthrene was used as a substrate. However, the surfactant inhibited the biotransformation of both naphthalene and phenanthrene with strain B8/36 under the same conditions. The observation that a nonionic surfactant could have such contrasting effects on PAH oxidation by different bacteria, which are known to be important for the degradation of these compounds in the environment, may explain why previous research on the application of the surfactants to PAH bioremediation has yielded inconclusive results. The surfactant inhibited growth of the wild-type strain S. yanoikuyae B1 on aromatic compounds but did not inhibit B8/36 dioxygenase enzyme activity in vitro.
Figures
Similar articles
-
Genetics of naphthalene and phenanthrene degradation by Comamonas testosteroni.J Ind Microbiol Biotechnol. 1997 Nov-Dec;19(5-6):401-7. doi: 10.1038/sj.jim.2900476. J Ind Microbiol Biotechnol. 1997. PMID: 9451837
-
Quantifying the biodegradation of phenanthrene by Pseudomonas stutzeri P16 in the presence of a nonionic surfactant.Appl Environ Microbiol. 1996 Jul;62(7):2387-92. doi: 10.1128/aem.62.7.2387-2392.1996. Appl Environ Microbiol. 1996. PMID: 8779577 Free PMC article.
-
Biodegradation of phenanthrene by Pseudomonas sp. strain PP2: novel metabolic pathway, role of biosurfactant and cell surface hydrophobicity in hydrocarbon assimilation.Appl Microbiol Biotechnol. 2003 May;61(4):342-51. doi: 10.1007/s00253-002-1218-y. Epub 2003 Feb 11. Appl Microbiol Biotechnol. 2003. PMID: 12743764
-
Anaerobic degradation of non-substituted aromatic hydrocarbons.Curr Opin Biotechnol. 2011 Jun;22(3):406-14. doi: 10.1016/j.copbio.2011.02.009. Epub 2011 Mar 11. Curr Opin Biotechnol. 2011. PMID: 21398107 Review.
-
Electrokinetic-Enhanced Remediation of Phenanthrene-Contaminated Soil Combined with Sphingomonas sp. GY2B and Biosurfactant.Appl Biochem Biotechnol. 2016 Apr;178(7):1325-38. doi: 10.1007/s12010-015-1949-8. Epub 2015 Dec 18. Appl Biochem Biotechnol. 2016. PMID: 26683200 Review.
Cited by
-
Whole cell microbial transformation in cloud point system.J Ind Microbiol Biotechnol. 2008 Jul;35(7):645-56. doi: 10.1007/s10295-008-0345-6. Epub 2008 Apr 8. J Ind Microbiol Biotechnol. 2008. PMID: 18392870 Review.
-
Bacterial properties changing under Triton X-100 presence in the diesel oil biodegradation systems: from surface and cellular changes to mono- and dioxygenases activities.Environ Sci Pollut Res Int. 2015 Mar;22(6):4305-15. doi: 10.1007/s11356-014-3668-z. Epub 2014 Oct 8. Environ Sci Pollut Res Int. 2015. PMID: 25292306 Free PMC article.
-
Uptake of Hydrocarbon by Pseudomonas fluorescens (P1) and Pseudomonas putida (K1) Strains in the Presence of Surfactants: A Cell Surface Modification.Water Air Soil Pollut. 2011 Jan;214(1-4):451-459. doi: 10.1007/s11270-010-0436-7. Epub 2010 May 19. Water Air Soil Pollut. 2011. PMID: 21258434 Free PMC article.
-
Biodegradation of 2-ethylhexyl nitrate by Mycobacterium austroafricanum IFP 2173.Appl Environ Microbiol. 2008 Oct;74(20):6187-93. doi: 10.1128/AEM.01142-08. Epub 2008 Aug 22. Appl Environ Microbiol. 2008. PMID: 18723659 Free PMC article.
-
Bioremediation of crude oil polluted soil by the white rot fungus, Pleurotus tuberregium (Fr.) Sing.Environ Sci Pollut Res Int. 2003;10(2):108-12. doi: 10.1065/espr2002.04.114. Environ Sci Pollut Res Int. 2003. PMID: 12729043
References
-
- Boyd D R, McMordie R A S, Sharma N D, Dalton H, Williams P, Jenkins R O. Stereospecific benzylic hydroxylation of bicyclic alkenes by Pseudomonas putida: isolation of (+)-R-1-hydroxy-1,2-dihydronaphthalene, an arene hydrate of naphthalene from metabolism of 1,2-dihydronaphthalene. J Chem Soc Chem Commun. 1989;1989:339–340.
-
- Boyd D R, Sharma N D, Agarwal R, Resnick S M, Shocken M J, Gibson D T, Sayer J M, Yagi H, Jerina D M. Bacterial dioxygenase-catalysed dihydrozylation and chemical resolution routes to enantiopure cis-dihydrodiols of chrysene. J Chem Soc Perkin Trans I. 1998;1998:1715–1723.
-
- Cerniglia C E. Biodegradation of polycylic aromatic hydrocarbons. Biodegradation. 1992;3:351–368.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

