Identification of MAGE-3 epitopes presented by HLA-DR molecules to CD4(+) T lymphocytes
- PMID: 10049940
- PMCID: PMC2192951
- DOI: 10.1084/jem.189.5.767
Identification of MAGE-3 epitopes presented by HLA-DR molecules to CD4(+) T lymphocytes
Abstract
MAGE-type genes are expressed by many tumors of different histological types and not by normal cells, except for male germline cells, which do not express major histocompatibility complex (MHC) molecules. Therefore, the antigens encoded by MAGE-type genes are strictly tumor specific and common to many tumors. We describe here the identification of the first MAGE-encoded epitopes presented by histocompatibility leukocyte antigen (HLA) class II molecules to CD4(+) T lymphocytes. Monocyte-derived dendritic cells were loaded with a MAGE-3 recombinant protein and used to stimulate autologous CD4(+) T cells. We isolated CD4(+) T cell clones that recognized two different MAGE-3 epitopes, MAGE-3114-127 and MAGE-3121-134, both presented by the HLA-DR13 molecule, which is expressed in 20% of Caucasians. The second epitope is also encoded by MAGE-1, -2, and -6. Our procedure should be applicable to other proteins for the identification of new tumor-specific antigens presented by HLA class II molecules. The knowledge of such antigens will be useful for evaluation of the immune response of cancer patients immunized with proteins or with recombinant viruses carrying entire genes coding for tumor antigens. The use of antigenic peptides presented by class II in addition to peptides presented by class I may also improve the efficacy of therapeutic antitumor vaccination.
Figures

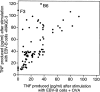

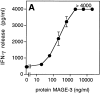




Comment in
-
CD4 T cells and their role in antitumor immune responses.J Exp Med. 1999 Mar 1;189(5):753-6. doi: 10.1084/jem.189.5.753. J Exp Med. 1999. PMID: 10049938 Free PMC article. No abstract available.
References
-
- Anichini A, Fossati G, Parmiani G. Clonal analysis of the cytolytic T-cell response to human tumors. Immunol Today. 1987;8:385–389. - PubMed
-
- Hérin M, Lemoine C, Weynants P, Vessière F, Van Pel A, Knuth A, Devos R, Boon T. Production of stable cytolytic T-cell clones directed against autologous human melanoma. Int J Cancer. 1987;39:390–396. - PubMed
-
- De Plaen E, Arden K, Traversari C, Gaforio JJ, Szikora J-P, De Smet C, Brasseur F, van der Bruggen P, Lethé B, Lurquin C, et al. Structure, chromosomal localization and expression of twelve genes of the MAGE family. Immunogenetics. 1994;40:360–369. - PubMed
-
- Van den Eynde B, van der Bruggen P. T cell defined tumor antigens. Curr Opin Immunol. 1997;9:684–693. - PubMed
-
- Takahashi K, Shichijo S, Noguchi M, Hirohata M, Itoh K. Identification of MAGE-1 and MAGE-4 proteins in spermatogonia and primary spermatocytes of testis. Cancer Res. 1995;55:3478–3482. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

