Calreticulin, a peptide-binding chaperone of the endoplasmic reticulum, elicits tumor- and peptide-specific immunity
- PMID: 10049943
- PMCID: PMC2192945
- DOI: 10.1084/jem.189.5.797
Calreticulin, a peptide-binding chaperone of the endoplasmic reticulum, elicits tumor- and peptide-specific immunity
Abstract
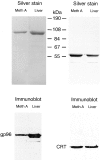
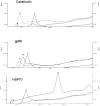
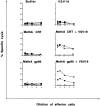
Calreticulin (CRT), a peptide-binding heat shock protein (HSP) of the endoplasmic reticulum (ER), has been shown previously to associate with peptides transported into the ER by transporter associated with antigen processing (Spee, P., and J. Neefjes. 1997. Eur. J. Immunol. 27: 2441-2449). Our studies show that CRT preparations purified from tumors elicit specific immunity to the tumor used as the source of CRT but not to an antigenically distinct tumor. The immunogenicity is attributed to the peptides associated with the CRT molecule and not to the CRT molecule per se. It is further shown that CRT molecules can be complexed in vitro to unglycosylated peptides and used to elicit peptide-specific CD8(+) T cell response in spite of exogenous administration. These characteristics of CRT closely resemble those of HSPs gp96, hsp90, and hsp70, although CRT has no apparent structural homologies to them.
Figures



References
-
- Srivastava PK, Das MR. The serologically unique cell surface antigen of Zajdela ascitic hepatoma is also its tumor-associated transplantation antigen. Int J Cancer. 1984;33:417–419. - PubMed
-
- Palladino MA, Srivastava PK, Oettgen HF, DeLeo AB. Expression of a shared tumor-specific antigen by two chemically induced BALB/c sarcomas. Cancer Res. 1987;47:5074–5079. - PubMed
-
- Udono H, Srivastava PK. Comparison of tumor-specific immunogenicities of stress-induced proteins gp96, hsp90, and hsp70. J Immunol. 1994;152:5398–5403. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

