Melanoma cells present a MAGE-3 epitope to CD4(+) cytotoxic T cells in association with histocompatibility leukocyte antigen DR11
- PMID: 10049951
- PMCID: PMC2192952
- DOI: 10.1084/jem.189.5.871
Melanoma cells present a MAGE-3 epitope to CD4(+) cytotoxic T cells in association with histocompatibility leukocyte antigen DR11
Abstract
In this study we used TEPITOPE, a new epitope prediction software, to identify sequence segments on the MAGE-3 protein with promiscuous binding to histocompatibility leukocyte antigen (HLA)-DR molecules. Synthetic peptides corresponding to the identified sequences were synthesized and used to propagate CD4(+) T cells from the blood of a healthy donor. CD4(+) T cells strongly recognized MAGE-3281-295 and, to a lesser extent, MAGE-3141-155 and MAGE-3146-160. Moreover, CD4(+) T cells proliferated in the presence of recombinant MAGE-3 after processing and presentation by autologous antigen presenting cells, demonstrating that the MAGE-3 epitopes recognized are naturally processed. CD4(+) T cells, mostly of the T helper 1 type, showed specific lytic activity against HLA-DR11/MAGE-3-positive melanoma cells. Cold target inhibition experiments demonstrated indeed that the CD4(+) T cells recognized MAGE-3281-295 in association with HLA-DR11 on melanoma cells. This is the first evidence that a tumor-specific shared antigen forms CD4(+) T cell epitopes. Furthermore, we validated the use of algorithms for the prediction of promiscuous CD4(+) T cell epitopes, thus opening the possibility of wide application to other tumor-associated antigens. These results have direct implications for cancer immunotherapy in the design of peptide-based vaccines with tumor-specific CD4(+) T cell epitopes.
Figures

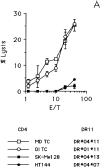

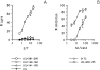
Comment in
-
CD4 T cells and their role in antitumor immune responses.J Exp Med. 1999 Mar 1;189(5):753-6. doi: 10.1084/jem.189.5.753. J Exp Med. 1999. PMID: 10049938 Free PMC article. No abstract available.
References
-
- Greenberg PD. Adoptive T cell therapy of tumors: mechanisms operative in the recognition and elimination of tumor cells. Adv Immunol. 1991;49:281–355. - PubMed
-
- Chen P, Aanathaswamy H. Rejection of K1735 murine melanoma in syngeneic hosts requires expression of MHC class I antigens and either class II antigens or IL-2. J Immunol. 1993;151:244–255. - PubMed
-
- Mandelboim O, Vadai E, Fridkin M, Katz-Hillel A, Feldman M, Berke G, Eisenbach L. Regression of established murine carcinoma metastases following vaccination with tumor-associated antigen peptides. Nat Med. 1995;1:1179–1183. - PubMed
-
- Mayordomo JI, Zorina T, Storkus WJ, Zitvogel L, Celluzzi C, Falo LD, Melief CJM, Ildstad ST, Kast WM, Deleo AB, Lotze MT. Bone marrow-derived dendritic cells pulsed with synthetic tumor peptides elicit protective and therapeutic antitumor immunity. Nat Med. 1995;1:1297–1302. - PubMed
-
- Bellone M, Iezzi G, Martin-Fontecha A, Rivolta L, Manfredi AA, Protti MP, Freschi M, Dellabona P, Casorati G, Rugarli C. Rejection of a non-immunogenic melanoma by vaccination with natural melanoma peptides on engineered APC. J Immunol. 1997;158:783–789. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

