Chloride dependence of hyperpolarization-activated chloride channel gates
- PMID: 10050002
- PMCID: PMC2269146
- DOI: 10.1111/j.1469-7793.1999.341ac.x
Chloride dependence of hyperpolarization-activated chloride channel gates
Abstract
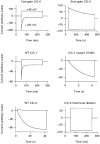
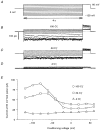
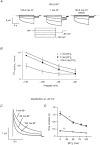
1. ClC proteins are a class of voltage-dependent Cl- channels with several members mutated in human diseases. The prototype ClC-0 Torpedo channel is a dimeric protein; each subunit forms a pore that can gate independently from the other one. A common slower gating mechanism acts on both pores simultaneously; slow gating activates ClC-0 at hyperpolarized voltages. The ClC-2 Cl- channel is also activated by hyperpolarization, as are some ClC-1 mutants (e.g. D136G) and wild-type (WT) ClC-1 at certain pH values. 2. We studied the dependence on internal Cl- ([Cl-]i) of the hyperpolarization-activated gates of several ClC channels (WT ClC-0, ClC-0 mutant P522G, ClC-1 mutant D136G and an N-terminal deletion mutant of ClC-2), by patch clamping channels expressed in Xenopus oocytes. 3. With all these channels, reducing [Cl-]i shifted activation to more negative voltages and reduced the maximal activation at most negative voltages. 4. We also investigated the external halide dependence of WT ClC-2 using two-electrode voltage-clamp recording. Reducing external Cl- ([Cl-]o) activated ClC-2 currents. Replacing [Cl-]o by the less permeant Br- reduced channel activity and accelerated deactivation. 5. Gating of the ClC-2 mutant K566Q in normal [Cl-]o resembled that of WT ClC-2 in low [Cl-]o, i.e. channels had a considerable open probability (Po) at resting membrane potential. Substituting external Cl- by Br- or I- led to a decrease in Po. 6. The [Cl-]i dependence of the hyperpolarization-activated gates of various ClC channels suggests a similar gating mechanism, and raises the possibility that the gating charge for the hyperpolarization-activated gate is provided by Cl-. 7. The external halide dependence of hyperpolarization-activated gating of ClC-2 suggests that it is mediated or modulated by anions as in other ClC channels. In contrast to the depolarization-activated fast gates of ClC-0 and ClC-1, the absence of Cl- favours channel opening. Lysine 556 may be important for the relevant binding site.
Figures


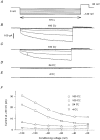
 . Both time constants decrease with increasing concentrations of bromide.
. Both time constants decrease with increasing concentrations of bromide.
References
-
- Carew MA, Thorn P. Identification of ClC-2-like chloride currents in pig pancreatic acinar cells. Pflügers Archiv. 1996;433:84–90. - PubMed
-
- Dinudom A, Young JA, Cook DI. Na+ and Cl− conductances are controlled by cytosolic Cl− concentration in the intralobular duct cells of mouse mandibular glands. Journal of Membrane Biology. 1993;135:289–295. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

