Depolarization-evoked Ca2+ release in a non-excitable cell, the rat megakaryocyte
- PMID: 10050006
- PMCID: PMC2269158
- DOI: 10.1111/j.1469-7793.1999.385ac.x
Depolarization-evoked Ca2+ release in a non-excitable cell, the rat megakaryocyte
Abstract
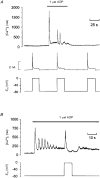
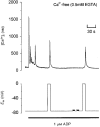
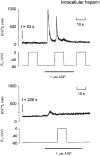
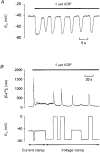
1. The effect of membrane potential on [Ca2+]i in rat megakaryocytes was studied using simultaneous whole-cell patch clamp and fura-2 fluorescence recordings. 2. Depolarization from -75 to 0 mV had no effect on [Ca2+]i in unstimulated cells, but evoked one or more spikes of Ca2+ increase (peak increase: 714 +/- 95 nM) during activation of metabotropic purinoceptors by 1 microM ADP. 3. The depolarization-evoked Ca2+ increase was present in Ca2+-free medium and also following removal of Na+. Thus depolarization mobilizes Ca2+ from an intracellular store without a requirement for altered Na+-Ca2+ exchange activity. 4. Intracellular dialysis with heparin blocked the depolarization-evoked Ca2+ increase, indicating a role for functional IP3 receptors. 5. Under current clamp, ADP caused the membrane potential to fluctuate between -43 +/- 1 and -76 +/- 1 mV. Under voltage clamp, depolarization from -75 to -45 mV evoked a transient [Ca2+]i increase (398 +/- 91 nM) during exposure to ADP. 6. We conclude that during stimulation of metabotropic purinoceptors, membrane depolarization over the physiological range can stimulate Ca2+ release from intracellular stores in the rat megakaryocyte, a non-excitable cell type. This may represent an important mechanism by which electrogenic influences can control patterns of [Ca2+]i increase.
Figures
Similar articles
-
A novel role for membrane potential in the modulation of intracellular Ca2+ oscillations in rat megakaryocytes.J Physiol. 2000 Apr 15;524 Pt 2(Pt 2):437-46. doi: 10.1111/j.1469-7793.2000.00437.x. J Physiol. 2000. PMID: 10766924 Free PMC article.
-
Three cation influx currents activated by purinergic receptor stimulation in rat megakaryocytes.J Physiol. 1994 Oct 15;480 ( Pt 2)(Pt 2):225-31. doi: 10.1113/jphysiol.1994.sp020355. J Physiol. 1994. PMID: 7532712 Free PMC article.
-
Cytoplasmic Ca2+ oscillation in rat megakaryocytes evoked by a novel type of purinoceptor.J Physiol. 1993 Oct;470:731-49. doi: 10.1113/jphysiol.1993.sp019885. J Physiol. 1993. PMID: 8308753 Free PMC article.
-
Voltage-dependent conductances of solitary ganglion cells dissociated from the rat retina.J Physiol. 1987 Apr;385:361-91. doi: 10.1113/jphysiol.1987.sp016497. J Physiol. 1987. PMID: 2443669 Free PMC article. Review.
-
Potassium-current oscillation of rat megakaryocytes: As a model system for drug evaluation (Review).Int J Mol Med. 1999 Aug;4(2):163-9. doi: 10.3892/ijmm.4.2.163. Int J Mol Med. 1999. PMID: 10402483 Review.
Cited by
-
A major interspecies difference in the ionic selectivity of megakaryocyte Ca2+-activated channels sensitive to the TMEM16F inhibitor CaCCinh-A01.Platelets. 2019;30(8):962-966. doi: 10.1080/09537104.2019.1595560. Epub 2019 Apr 22. Platelets. 2019. PMID: 31008669 Free PMC article.
-
The interpretation of current-clamp recordings in the cell-attached patch-clamp configuration.Biophys J. 2005 Jan;88(1):739-50. doi: 10.1529/biophysj.104.049866. Epub 2004 Oct 29. Biophys J. 2005. PMID: 15516522 Free PMC article.
-
Pacing of interstitial cells of Cajal in the murine gastric antrum: neurally mediated and direct stimulation.J Physiol. 2003 Dec 1;553(Pt 2):545-59. doi: 10.1113/jphysiol.2003.050419. Epub 2003 Sep 18. J Physiol. 2003. PMID: 14500772 Free PMC article.
-
A study of P2X1 receptor function in murine megakaryocytes and human platelets reveals synergy with P2Y receptors.Br J Pharmacol. 2002 Jan;135(2):363-72. doi: 10.1038/sj.bjp.0704486. Br J Pharmacol. 2002. PMID: 11815371 Free PMC article.
-
Sensitivity limits for voltage control of P2Y receptor-evoked Ca2+ mobilization in the rat megakaryocyte.J Physiol. 2004 Feb 15;555(Pt 1):61-70. doi: 10.1113/jphysiol.2003.056846. Epub 2003 Nov 28. J Physiol. 2004. PMID: 14645457 Free PMC article.
References
-
- Bezprozvanny I, Ehrlich BE. The inositol 1,4,5-trisphosphate (InsP3) receptor. Journal of Membrane Biology. 1995;145:205–216. - PubMed
-
- Clapham DE. Calcium signaling. Cell. 1995;80:259–268. - PubMed
-
- Fasolato C, Innocenti B, Pozzan T. Receptor-activated Ca2+ influx: how many mechanisms for how many channels? Trends in Pharmacological Sciences. 1994;15:77–83. 10.1016/0165-6147(94)90282-8. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

