Acetylcholine-induced membrane potential changes in endothelial cells of rabbit aortic valve
- PMID: 10051116
- PMCID: PMC1565773
- DOI: 10.1038/sj.bjp.0702262
Acetylcholine-induced membrane potential changes in endothelial cells of rabbit aortic valve
Abstract
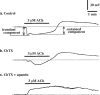
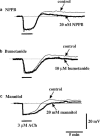
1. Using a microelectrode technique, acetylcholine (ACh)-induced membrane potential changes were characterized using various types of inhibitors of K+ and Cl- channels in rabbit aortic valve endothelial cells (RAVEC). 2. ACh produced transient then sustained membrane hyperpolarizations. Withdrawal of ACh evoked a transient depolarization. 3. High K+ blocked and low K+ potentiated the two ACh-induced hyperpolarizations. Charybdotoxin (ChTX) attenuated the ACh-induced transient and sustained hyperpolarizations; apamin inhibited only the sustained hyperpolarization. In the combined presence of ChTX and apamin, ACh produced a depolarization. 4. In Ca2+-free solution or in the presence of Co2+ or Ni2+, ACh produced a transient hyperpolarization followed by a depolarization. In BAPTA-AM-treated cells, ACh produced only a depolarization. 5. A low concentration of A23187 attenuated the ACh-induced transient, but not the sustained, hyperpolarization. In the presence of cyclopiazonic acid, the hyperpolarization induced by ACh was maintained after ACh removal; this maintained hyperpolarization was blocked by Co2+. 6. Both NPPB and hypertonic solution inhibited the membrane depolarization seen after ACh washout. Bumetanide also attenuated this depolarization. 7. It is concluded that in RAVEC, ACh produces a two-component hyperpolarization followed by a depolarization. It is suggested that ACh-induced Ca2+ release from the storage sites causes a transient hyperpolarization due to activation of ChTX-sensitive K+ channels and that ACh-activated Ca2+ influx causes a sustained hyperpolarization by activating both ChTX- and apamin-sensitive K+ channels. Both volume-sensitive Cl- channels and the Na+-K+-Cl- cotransporter probably contribute to the ACh-induced depolarization.
Figures




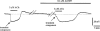
Similar articles
-
Effects of increased intracellular Cl- concentration on membrane responses to acetylcholine in the isolated endothelium of guinea pig mesenteric arteries.J Physiol Sci. 2007 Feb;57(1):31-41. doi: 10.2170/physiolsci.RP012606. Epub 2006 Dec 28. J Physiol Sci. 2007. PMID: 17190590
-
Reduced hyperpolarization in endothelial cells of rabbit aortic valve following chronic nitroglycerine administration.Br J Pharmacol. 2005 Oct;146(4):487-97. doi: 10.1038/sj.bjp.0706363. Br J Pharmacol. 2005. PMID: 16056232 Free PMC article.
-
Cellular target of voltage and calcium-dependent K(+) channel blockers involved in EDHF-mediated responses in rat superior mesenteric artery.Br J Pharmacol. 2001 Nov;134(5):1021-8. doi: 10.1038/sj.bjp.0704348. Br J Pharmacol. 2001. PMID: 11682450 Free PMC article.
-
Apamin/charybdotoxin-sensitive endothelial K+ channels contribute to acetylcholine-induced, NO-dependent vasorelaxation of rat aorta.Med Sci Monit. 2001 Nov-Dec;7(6):1129-36. Med Sci Monit. 2001. PMID: 11687720
-
Electrophysiological properties of gastric pacemaker potentials.J Smooth Muscle Res. 2003 Oct;39(5):163-73. doi: 10.1540/jsmr.39.163. J Smooth Muscle Res. 2003. PMID: 14695027 Review.
Cited by
-
Endothelium-dependent smooth muscle hyperpolarization: do gap junctions provide a unifying hypothesis?Br J Pharmacol. 2004 Mar;141(6):881-903. doi: 10.1038/sj.bjp.0705698. Br J Pharmacol. 2004. PMID: 15028638 Free PMC article. Review.
-
K+ currents underlying the action of endothelium-derived hyperpolarizing factor in guinea-pig, rat and human blood vessels.J Physiol. 2001 Mar 1;531(Pt 2):359-73. doi: 10.1111/j.1469-7793.2001.0359i.x. J Physiol. 2001. PMID: 11230509 Free PMC article.
-
Gap junctions in the control of vascular function.Antioxid Redox Signal. 2009 Feb;11(2):251-66. doi: 10.1089/ars.2008.2117. Antioxid Redox Signal. 2009. PMID: 18831678 Free PMC article. Review.
-
Characteristics of ACh-induced hyperpolarization and relaxation in rabbit jugular vein.Br J Pharmacol. 2012 Oct;167(3):682-96. doi: 10.1111/j.1476-5381.2012.02038.x. Br J Pharmacol. 2012. PMID: 22595036 Free PMC article.
-
The uptake and metabolism of fructose-1,6-diphosphate in rat cardiomyocytes.Mol Cell Biochem. 2001 May;221(1-2):33-40. doi: 10.1023/a:1010973806747. Mol Cell Biochem. 2001. PMID: 11506184
References
-
- BÉNY J.-L., PACICCA C. Bidirectional electrical communication between smooth muscle and endothelial cells in the pig coronary artery. Am. J. Physiol. 1994;266:H1465–H1472. - PubMed
-
- BÉNY J.-L. Electrical coupling between smooth muscle cells and endothelial cells in pig coronary arteries. Pflügers Arch. 1997;433:364–367. - PubMed
-
- BRUNET P.C., BÉNY J.-L. Substance P and bradykinin hyperpolarize pig coronary artery endothelial cells in primary culture. Blood Vessels. 1989;26:228–234. - PubMed
-
- CABANTCHIK Z.I., GREGER R. Chemical probes for anion transporters of mammalian cell membranes. Am. J. Physiol. 1992;262:C803–C827. - PubMed
-
- CARTER T.D., OGDEN D. Acetylcholine-stimulated changes of membrane potential and intracellular Ca2+ concentration recorded in endothelial cells in situ in the isolated rat aorta. Pflügers Arch. 1994;428:476–484. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

