Modulation of chloride, potassium and bicarbonate transport by muscarinic receptors in a human adenocarcinoma cell line
- PMID: 10051145
- PMCID: PMC1565781
- DOI: 10.1038/sj.bjp.0702270
Modulation of chloride, potassium and bicarbonate transport by muscarinic receptors in a human adenocarcinoma cell line
Abstract
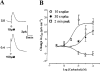
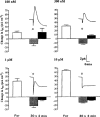
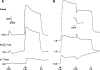
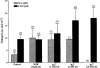
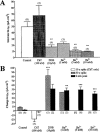
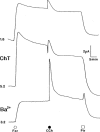
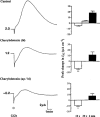
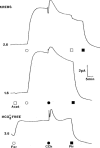
1. Short-circuit current (I(SC)) responses to carbachol (CCh) were investigated in Colony 1 epithelia, a subpopulation of the HCA-7 adenocarcinoma cell line. In Krebs-Henseleit (KH) buffer, CCh responses consisted of three I(SC) components: an unusual rapid decrease (the 10 s spike) followed by an upward spike at 30 s and a slower transient increase (the 2 min peak). This response was not potentiated by forskolin; rather, CCh inhibited cyclic AMP-stimulated I(SC). 2. In HCO3- free buffer, the decrease in forskolin-elevated I(SC) after CCh was reduced, although the interactions between CCh and forskolin remained at best additive rather than synergistic. When Cl- anions were replaced by gluconate, both Ca2+- and cyclic AMP-mediated electrogenic responses were significantly inhibited. 3. Basolateral Ba2+ (1-10 mM) and 293B (10 microM) selectively inhibited forskolin stimulation of I(SC), without altering the effects of CCh. Under Ba2+- or 293B-treated conditions, CCh responses were potentiated by pretreatment with forskolin. 4. Basolateral charybdotoxin (50 nM) significantly increased the size of the 10 s spike of CCh responses in both KH and HCO3- free medium, without affecting the 2 min peak. The enhanced 10 s spike was inhibited by prior addition of 5 mM apical Ba2+. Charybdotoxin did not affect forskolin responses. 5. In epithelial layers prestimulated with forskolin, the muscarinic antagonists atropine and 4-diphenylacetoxy-N-methylpiperidine methiodide (4-DAMP, both at 100 nM) abolished subsequent 10 microM CCh responses. Following addition of p-fluoro hexahydro-sila-difenidol (pF-HHSiD, 10 microM) or pirenzepine (1 microM), qualitative changes in the CCh response time-profile also indicated a rightward shift of the agonist concentration-response curve; however, 1 microM gallamine had no effect. These results suggest that a single M3-like receptor subtype mediates the secretory response to CCh. 6. It is concluded that CCh and forskolin activate discrete populations of basolateral K+ channels gated by either Ca2+ or cyclic AMP, but that the Cl- permeability of the apical membrane may limit their combined effects on electrogenic Cl- secretion. In addition, CCh activates a Ba2+-sensitive apical K+ conductance leading to electrogenic K+ transport. Both agents may also modulate HCO3- secretion through a mechanism at least partially dependent on carbonic anhydrase.
Figures


References
-
- BAJNATH R.B., DEKKER K., VAANDRAGER A.B., DE JONGE H.R., GROOT J.A. Biphasic increase of apical Cl− conductance by muscarinic stimulation of HT-29cl.19A human colon carcinoma cell line: evidence for activation of different Cl− conductances by carbachol and forskolin. J. Membr. Biol. 1992;127:81–94. - PubMed
-
- BINDER H.J., SANDLE G.I.Electrolyte transport in the mammalian colon Physiology of the Gastrointestinal Tract 1994New York, Raven Press; 2133–2171.ed. Johnson, L.R., Alpers, D.H., Christensen, J., Jacobsen, E.D. & Walsh, J.H. pp
-
- BLEICH M., RIEDEMANN N., WARTH R., KERSTAN D., LEIPZIGER J., HÖR M., VAN DRIESSCHE W., GREGER R. Ca2+ regulated K+ and non-selective cation channels in the basolateral membrane of rat colonic crypt base cells. Pflügers Arch. 1996;432:1011–1022. - PubMed
-
- BORNSTEIN J.C., FURNESS J.B. Correlated electrophysical and histo-chemical studies of submucous neurons and their contribution to understanding enteric neuronal circuits. J. Autonom. Nerv. System. 1988;25:1–13. - PubMed
-
- BUSCH A.E., SUESSBRICH H. Role of the IsK protein in the IminK channel complex. TiPs. 1997;18:26–29. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

