Multiple genetic modifications of the erythromycin polyketide synthase to produce a library of novel "unnatural" natural products
- PMID: 10051557
- PMCID: PMC26699
- DOI: 10.1073/pnas.96.5.1846
Multiple genetic modifications of the erythromycin polyketide synthase to produce a library of novel "unnatural" natural products
Erratum in
- 2000 Apr 25;97(9):5011
- Proc Natl Acad Sci U S A 1999 May 11;96(10):5890
Abstract
The structures of complex polyketide natural products, such as erythromycin, are programmed by multifunctional polyketide synthases (PKSs) that contain modular arrangements of functional domains. The colinearity between the activities of modular PKS domains and structure of the polyketide product portends the generation of novel organic compounds-"unnatural" natural products-by genetic manipulation. We have engineered the erythromycin polyketide synthase genes to effect combinatorial alterations of catalytic activities in the biosynthetic pathway, generating a library of >50 macrolides that would be impractical to produce by chemical methods. The library includes examples of analogs with one, two, and three altered carbon centers of the polyketide products. The manipulation of multiple biosynthetic steps in a PKS is an important milestone toward the goal of producing large libraries of unnatural natural products for biological and pharmaceutical applications.
Figures
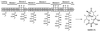

Comment in
-
Microbial polyketide synthases: more and more prolific.Proc Natl Acad Sci U S A. 1999 Mar 30;96(7):3336-8. doi: 10.1073/pnas.96.7.3336. Proc Natl Acad Sci U S A. 1999. PMID: 10097038 Free PMC article. Review. No abstract available.
References
-
- Omura S. Gene. 1992;115:141–149. - PubMed
-
- Zinner S H, Young L S, Acar J F, Neu H C. Expanding Indications for the New Macrolides, Azalides, and Streptogramins. New York: Dekker; 1997.
-
- Woodward R B, Logusch E, Nambiar K P, Sakan K, Ward D E, Au-Yeung B W, Balaram P, Browne L J, Card P J, Chen C H, et al. J Am Chem Soc. 1981;103:3215–3217.
-
- Chu D T W. Exp Opin Invest Drugs. 1995;4:65–94.
-
- Cortés J, Haydock S F, Roberts G A, Bevitt D J, Leadlay P F. Nature (London) 1990;348:176–178. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

