The GTPase activating factor for transducin in rod photoreceptors is the complex between RGS9 and type 5 G protein beta subunit
- PMID: 10051575
- PMCID: PMC26717
- DOI: 10.1073/pnas.96.5.1947
The GTPase activating factor for transducin in rod photoreceptors is the complex between RGS9 and type 5 G protein beta subunit
Abstract
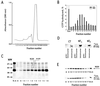
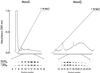
Proteins of the regulators of G protein signaling (RGS) family modulate the duration of intracellular signaling by stimulating the GTPase activity of G protein alpha subunits. It has been established that the ninth member of the RGS family (RGS9) participates in accelerating the GTPase activity of the photoreceptor-specific G protein, transducin. This process is essential for timely inactivation of the phototransduction cascade during the recovery from a photoresponse. Here we report that functionally active RGS9 from vertebrate photoreceptors exists as a tight complex with the long splice variant of the G protein beta subunit (Gbeta5L). RGS9 and Gbeta5L also form a complex when coexpressed in cell culture. Our data are consistent with the recent observation that several RGS proteins, including RGS9, contain G protein gamma-subunit like domain that can mediate their association with Gbeta5 (Snow, B. E., Krumins, A. M., Brothers, G. M., Lee, S. F., Wall, M. A., Chung, S., Mangion, J., Arya, S., Gilman, A. G. & Siderovski, D. P. (1998) Proc. Natl. Acad. Sci. USA 95, 13307-13312). We report an example of such a complex whose cellular localization and function are clearly defined.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

