Tolerance of Arc repressor to multiple-alanine substitutions
- PMID: 10051581
- PMCID: PMC26723
- DOI: 10.1073/pnas.96.5.1983
Tolerance of Arc repressor to multiple-alanine substitutions
Abstract
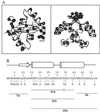
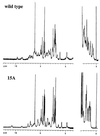
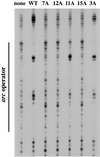
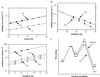
Arc repressor mutants containing from three to 15 multiple-alanine substitutions have spectral properties expected for native Arc proteins, form heterodimers with wild-type Arc, denature cooperatively with Tms equal to or greater than wild type, and, in some cases, fold as much as 30-fold faster and unfold as much as 50-fold slower than wild type. Two of the mutants, containing a total of 14 different substitutions, also footprint operator DNA in vitro. The stability of some of the proteins with multiple-alanine mutations is significantly greater than that predicted from the sum of the single substitutions, suggesting that a subset of the wild-type residues in Arc may interact in an unfavorable fashion. Overall, these results show that almost half of the residues in Arc can be replaced by alanine en masse without compromising the ability of this small, homodimeric protein to fold into a stable, native-like structure.
Figures

References
-
- Bowie J U, Reidhaar-Olson J F, Lim W A, Sauer R T. Science. 1990;247:1306–1310. - PubMed
-
- Rennell D, Bouvier S, Hardy L, Poteete A R. J Mol Biol. 1991;222:67–87. - PubMed
-
- Basse W A, Eriksson A E, Zhang X J, Heinz D W, Sauer U, Blaber M, Baldwin E P, Wozniak J A, Matthews B W. Faraday Discuss Chem Soc. 1992;93:173–181. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

