Immunophilins, Refsum disease, and lupus nephritis: the peroxisomal enzyme phytanoyl-COA alpha-hydroxylase is a new FKBP-associated protein
- PMID: 10051602
- PMCID: PMC26744
- DOI: 10.1073/pnas.96.5.2104
Immunophilins, Refsum disease, and lupus nephritis: the peroxisomal enzyme phytanoyl-COA alpha-hydroxylase is a new FKBP-associated protein
Abstract
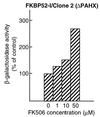
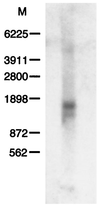
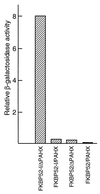
FKBP52 (FKBP59, FKBP4) is a "macro" immunophilin that, although sharing high structural and functional homologies in its amino-terminal domain with FKBP12 (FKBP1), does not have immunosuppressant activity when complexed with FK506, unlike FKBP12. To investigate the physiological function of FKBP52, we used the yeast two-hybrid system as an approach to find its potential protein partners and, from that, its cellular role. This methodology, which already has allowed us to find the FK506-binding protein (FKBP)-associated protein FAP48, also led to the detection of another FKBP-associated protein. Determination of the sequence of this protein permitted its identification as phytanoyl-CoA alpha-hydroxylase (PAHX), a peroxisomal enzyme that so far was unknown as an FKBP-associated protein. Inactivation of this enzyme is responsible for Refsum disease in humans. The protein also corresponds to the mouse protein LN1, which could be involved in the progress of lupus nephritis. We show here that PAHX has the physical capacity to interact with the FKBP12-like domain of FKBP52, but not with FKBP12, suggesting that it is a particular and specific target of FKBP52. Whereas the binding of calcineurin to FKBP12 is potentiated by FK506, the specific association of PAHX and FKBP52 is maintained in the presence of FK506. This observation suggests that PAHX is a serious candidate for studying the cellular signaling pathway(s) involving FKBP52 in the presence of immunosuppressant drugs.
Figures


References
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

