Regulation of G1 progression by the PTEN tumor suppressor protein is linked to inhibition of the phosphatidylinositol 3-kinase/Akt pathway
- PMID: 10051603
- PMCID: PMC26745
- DOI: 10.1073/pnas.96.5.2110
Regulation of G1 progression by the PTEN tumor suppressor protein is linked to inhibition of the phosphatidylinositol 3-kinase/Akt pathway
Abstract
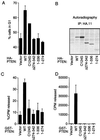
PTEN/MMAC1 is a tumor suppressor gene located on chromosome 10q23. Inherited PTEN/MMAC1 mutations are associated with a cancer predisposition syndrome known as Cowden's disease. Somatic mutation of PTEN has been found in a number of malignancies, including glioblastoma, melanoma, and carcinoma of the prostate and endometrium. The protein product (PTEN) encodes a dual-specificity protein phosphatase and in addition can dephosphorylate certain lipid substrates. Herein, we show that PTEN protein induces a G1 block when reconstituted in PTEN-null cells. A PTEN mutant associated with Cowden's disease (PTEN;G129E) has protein phosphatase activity yet is defective in dephosphorylating inositol 1,3,4,5-tetrakisphosphate in vitro and fails to arrest cells in G1. These data suggest a link between induction of a cell-cycle block by PTEN and its ability to dephosphorylate, in vivo, phosphatidylinositol 3,4,5-trisphosphate. In keeping with this notion, PTEN can inhibit the phosphatidylinositol 3,4, 5-trisphosphate-dependent Akt kinase, a downstream target of phosphatidylinositol 3-kinase, and constitutively active, but not wild-type, Akt overrides a PTEN G1 arrest. Finally, tumor cells lacking PTEN contain high levels of activated Akt, suggesting that PTEN is necessary for the appropriate regulation of the phosphatidylinositol 3-kinase/Akt pathway.
Figures
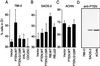
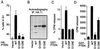


References
-
- Gray I C, Phillips S M, Lee S J, Neoptolemos J P, Weissenbach J, Spurr N K. Cancer Res. 1995;55:4800–4803. - PubMed
-
- Rasheed B K, McLendon R E, Friedman H S, Friedman A H, Fuchs H E, Bigner D D, Bigner S H. Oncogene. 1995;10:2243–2246. - PubMed
-
- Steck P A, Pershouse M A, Jasser S A, Yung W K, Lin H, Ligon A H, Langford L A, Baumgard M L, Hattier T, Davis T, et al. Nat Genet. 1997;15:356–362. - PubMed
-
- Li J, Yen C, Liaw D, Podsypanina K, Bose S, Wang S I, Puc J, Miliaresis C, Rodgers L, McCombie R, et al. Science. 1997;275:1943–1947. - PubMed
-
- Li D M, Sun H. Cancer Res. 1997;57:2124–2129. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

