Dynamic and quantitative Ca2+ measurements using improved cameleons
- PMID: 10051607
- PMCID: PMC26749
- DOI: 10.1073/pnas.96.5.2135
Dynamic and quantitative Ca2+ measurements using improved cameleons
Abstract
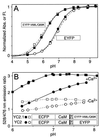
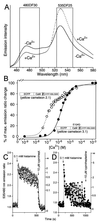
Cameleons are genetically-encoded fluorescent indicators for Ca2+ based on green fluorescent protein variants and calmodulin (CaM). Because cameleons can be targeted genetically and imaged by one- or two-photon excitation microscopy, they offer great promise for monitoring Ca2+ in whole organisms, tissues, organelles, and submicroscopic environments in which measurements were previously impossible. However, the original cameleons suffered from significant pH interference, and their Ca2+-buffering and cross-reactivity with endogenous CaM signaling pathways was uncharacterized. We have now greatly reduced the pH-sensitivity of the cameleons by introducing mutations V68L and Q69K into the acceptor yellow green fluorescent protein. The resulting new cameleons permit Ca2+ measurements despite significant cytosolic acidification. When Ca2+ is elevated, the CaM and CaM-binding peptide fused together in a cameleon predominantly interact with each other rather than with free CaM and CaM-dependent enzymes. Therefore, if cameleons are overexpressed, the primary effect is likely to be the unavoidable increase in Ca2+ buffering rather than specific perturbation of CaM-dependent signaling.
Figures



References
-
- Tsien R Y. Annu Rev Neurosci. 1989;12:227–253. - PubMed
-
- Brini M, Marsault R, Bastianutto C, Alvarez J, Pozzan T, Rizzuto R. J Biol Chem. 1995;270:9896–9903. - PubMed
-
- Miyawaki A, Llopis J, Heim R, McCaffery J M, Adams J A, Ikura M, Tsien R Y. Nature (London) 1997;388:882–887. - PubMed
-
- Porumb T, Yau P, Harvey T S, Ikura M. Protein Eng. 1994;7:109–115. - PubMed
-
- Ikura M, Clore G M, Gronenborn A M, Zhu G, Klee C B, Bax A. Science. 1992;256:632–638. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

