Immune surveillance against a solid tumor fails because of immunological ignorance
- PMID: 10051624
- PMCID: PMC26766
- DOI: 10.1073/pnas.96.5.2233
Immune surveillance against a solid tumor fails because of immunological ignorance
Abstract
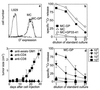
Many peripheral solid tumors such as sarcomas and carcinomas express tumor-specific antigens that can serve as targets for immune effector T cells. Nevertheless, overall immune surveillance against such tumors seems relatively inefficient. We studied immune surveillance against a s.c. sarcoma expressing a characterized viral tumor antigen. Surprisingly, the tumor cells were capable of inducing a protective cytotoxic T cell response if transferred as a single-cell suspension. However, if they were transplanted as small tumor pieces, tumors readily grew. Tumor growth correlated strictly with (i) failure of tumor cells to reach the draining lymph nodes and (ii) absence of primed cytotoxic T cells. Cytotoxic T cells were not tolerant or deleted because a tumor antigen-specific cytotoxic T cell response was readily induced in lymphoid tissue by immunization with virus or with tumor cells even in the presence of large tumors. Established tumors were rejected by vaccine-induced effector T cells if effector T cells were maintained by prolonged or repetitive vaccination, but not by single-dose vaccination. Thus, in addition to several other tumor-promoting parameters, some antigenic peripheral sarcomas-and probably carcinomas-may grow not because they anergize or tolerize tumor-specific T cells, but because such tumors are immunologically dealt with as if they were in a so-called immunologically privileged site and are ignored for too long.
Figures
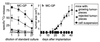



References
-
- Old L J, Boyse E A, Clarke D A. Ann N Y Acad Sci. 1962;101:80–106.
-
- Pardoll D M. Nat Med. 1998;4:525–531. - PubMed
-
- Boon T, Cerottini J C, van-den-Eynde B, van-der-Bruggen P, Van-Pel A. Annu Rev Immunol. 1994;12:337–365. - PubMed
-
- Möller G. Immunol Rev. 1995;145:1–250.
-
- Andrews E J. J Natl Cancer Inst. 1974;52:729–732. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

