The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1
- PMID: 10051653
- PMCID: PMC26795
- DOI: 10.1073/pnas.96.5.2396
The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1
Abstract
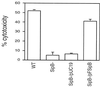
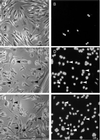
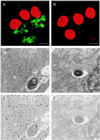
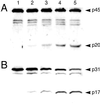
Recently, Salmonella spp. were shown to induce apoptosis in infected macrophages. The mechanism responsible for this process is unknown. In this report, we establish that the Inv-Spa type III secretion apparatus target invasin SipB is necessary and sufficient for the induction of apoptosis. Purified SipB microinjected into macrophages led to cell death. Binding studies show that SipB associates with the proapoptotic protease caspase-1. This interaction results in the activation of caspase-1, as seen in its proteolytic maturation and the processing of its substrate interleukin-1beta. Caspase-1 activity is essential for the cytotoxicity. Functional inhibition of caspase-1 activity by acetyl-Tyr-Val-Ala-Asp-chloromethyl ketone blocks macrophage cytotoxicity, and macrophages lacking caspase-1 are not susceptible to Salmonella-induced apoptosis. Taken together, the data demonstrate that SipB functions as an analog of the Shigella invasin IpaB.
Figures
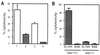
References
-
- Keusch G T. In: Harrison’s Principles of Internal Medicine. 13th Ed. Isselbacher K J, Braunwald E, Wilson J D, Martin J B, Fauci A S, Kasper D L, editors. New York: McGraw–Hill; 1994. pp. 671–676.
-
- Finlay B B. Curr Top Microbiol Immunol. 1994;192:163–185. - PubMed
-
- Clark M A, Jepson M A, Simmons N L, Hirst B H. Res Microbiol. 1994;145:543–553. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

