Bilirubin, formed by activation of heme oxygenase-2, protects neurons against oxidative stress injury
- PMID: 10051662
- PMCID: PMC26804
- DOI: 10.1073/pnas.96.5.2445
Bilirubin, formed by activation of heme oxygenase-2, protects neurons against oxidative stress injury
Erratum in
- Proc Natl Acad Sci U S A 1999 Sep 14;96(19):10944
Abstract
Heme oxygenase (HO) catalyzes the conversion of heme to carbon monoxide, iron, and biliverdin, which is immediately reduced to bilirubin (BR). Two HO active isozymes exist: HO1, an inducible heat shock protein, and HO2, which is constitutive and highly concentrated in neurons. We demonstrate a neuroprotective role for BR formed from HO2. Neurotoxicity elicited by hydrogen peroxide in hippocampal and cortical neuronal cultures is prevented by the phorbol ester, phorbol 12-myristate 13-acetate (PMA) via stimulation of protein kinase C. We observe phosphorylation of HO2 through the protein kinase C pathway with enhancement of HO2 catalytic activity and accumulation of BR in neuronal cultures. The neuroprotective effects of PMA are prevented by the HO inhibitor tin protoporphyrin IX and in cultures from mice with deletion of HO2 gene. Moreover, BR, an antioxidant, is neuroprotective at nanomolar concentrations.
Figures



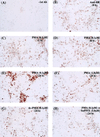
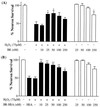
References
-
- Maines M D. Annu Rev Pharmacol Toxicol. 1997;37:517–554. - PubMed
-
- Ewing J F, Maines M D. Brain Res Brain Res Protoc. 1997;1:165–174. - PubMed
-
- Verma A, Hirsch D J, Glatt C E, Ronnett G V, Snyder S H. Science. 1993;259:381–384. - PubMed
-
- Stocker R, Yamamoto Y, McDonagh A F, Glazer A N, Ames B N. Science. 1987;235:1043–1046. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

