Efficacy and safety of rivastigmine in patients with Alzheimer's disease: international randomised controlled trial
- PMID: 10066203
- PMCID: PMC27767
- DOI: 10.1136/bmj.318.7184.633
Efficacy and safety of rivastigmine in patients with Alzheimer's disease: international randomised controlled trial
Erratum in
- BMJ 2001 Jun 16;322(7300):1456
Abstract
Objectives: To assess the effects of rivastigmine on the core domains of Alzheimer's disease.
Design: Prospective, randomised, multicentre, double blind, placebo controlled, parallel group trial. Patients received either placebo, 1-4 mg/day (lower dose) rivastigmine, or 6-12 mg/day (higher dose) rivastigmine. Doses were increased in one of two fixed dose ranges (1-4 mg/day or 6-12 mg/day) over the first 12 weeks with a subsequent assessment period of 14 weeks.
Setting: 45 centres in Europe and North America.
Participants: 725 patients with mild to moderately severe probable Alzheimer's disease diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders, fourth edition, and the criteria of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer's Disease and Related Disorders Association.
Outcome measures: Cognitive subscale of the Alzheimer's disease assessment scale, rating on the clinician interview based impression of change incorporating caregiver information scale, and the progressive deterioration scale.
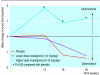
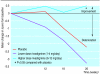
Results: At the end of the study cognitive function had deteriorated among those in the placebo group. Scores on the Alzheimer's disease assessment scale improved in patients in the higher dose group when compared with patients taking placebo (P<0.05). Significantly more patients in the higher dose group had improved by 4 points or more than had improved in the placebo group (24% (57/242) v 16% (39/238)). Global function as rated by the clinician interview scale had significantly improved among those in the higher dose group compared with those taking placebo (P<0.001), and significantly more patients in the higher dose group showed improvement than did in the placebo group (37% (80/219) v 20% (46/230)). Mean scores on the progressive deterioration scale improved from baseline in patients in the higher dose group but fell in the placebo group. Adverse events were predominantly gastrointestinal, of mild to moderate severity, transient, and occurred mainly during escalation of the dose. 23% (55/242) of those in the higher dose group, 7% (18/242) of those in the lower dose group, and 7% (16/239) of those in the placebo group discontinued treatment because of adverse events.
Conclusions: Rivastigmine is well tolerated and effective. It improves cognition, participation in activities of daily living, and global evaluation ratings in patients with mild to moderately severe Alzheimer's disease. This is the first treatment to show compelling evidence of efficacy in a predominantly European population.
Figures

Comment in
-
Acetylcholinesterase inhibitors for Alzheimer's disease.BMJ. 1999 Mar 6;318(7184):615-6. doi: 10.1136/bmj.318.7184.615. BMJ. 1999. PMID: 10066180 Free PMC article. No abstract available.
-
Commentary: Another piece of the Alzheimer's jigsaw.BMJ. 1999 Mar 6;318(7184):639. BMJ. 1999. PMID: 10215360 No abstract available.
-
Effectiveness of rivastigmine in Alzheimer's disease. Improvements in functional ability remain unestablished.BMJ. 1999 Sep 4;319(7210):640-1. doi: 10.1136/bmj.319.7210.640a. BMJ. 1999. PMID: 10473490 Free PMC article. No abstract available.
-
Effectiveness of rivastigmine in Alzheimer's disease. Patients' view on quality of life should be assessed.BMJ. 1999 Sep 4;319(7210):641-2. BMJ. 1999. PMID: 10523095 No abstract available.
-
Effectiveness of rivastigmine in Alzheimer's disease. Guidelines do not ignore clinically relevant end points.BMJ. 1999 Sep 4;319(7210):642. BMJ. 1999. PMID: 10523096 No abstract available.
Comment on
-
Science commentary: rational drug design for Alzheimer's disease.BMJ. 1999 Mar 6;318(7184):639. BMJ. 1999. PMID: 10215361 No abstract available.
References
-
- Knapp MJ, Knopman DS, Solomon PR, Pendlebury WW, Davis CS, Gracon SI, et al. A 30-week randomized controlled trial of high-dose tacrine in patients with Alzheimer’s disease. JAMA. 1994;271:985–991. - PubMed
-
- Davis KL, Thal LJ, Gamzu ER, Davis CS, Woolson RF, Gracon SI, et al. A double-blind, placebo-controlled multicenter study of tacrine for Alzheimer’s disease. N Engl J Med. 1992;327:1253–1259. - PubMed
-
- Farlow M, Gracon SI, Hershey LA, Lewis KW, Sadowsky CH, Dolan-Ureno J. A controlled trial of tacrine in Alzheimer’s disease. JAMA. 1992;268:2523–2529. - PubMed
-
- Roger SL, Farlow MR, Doody RS, Mohs R, Friedhoff LT. A 24-week, double-blind, placebo controlled trial of donepezil in patients with Alzheimer’s disease. Neurology. 1998;50:136–145. - PubMed
-
- Rogers SL, Friedhoff LT. The efficacy and safety of donepezil in patients with Alzheimer’s disease: results of a US multicentre, randomized, double-blind, placebo-controlled trial. Dementia. 1996;7:293–303. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
