Differential distribution of three members of a gene family encoding low voltage-activated (T-type) calcium channels
- PMID: 10066243
- PMCID: PMC6782581
- DOI: 10.1523/JNEUROSCI.19-06-01895.1999
Differential distribution of three members of a gene family encoding low voltage-activated (T-type) calcium channels
Abstract
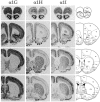
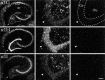
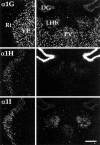
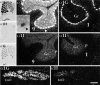
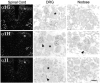
Low voltage-activated (T-type) calcium currents are observed in many central and peripheral neurons and display distinct physiological and functional properties. Using in situ hybridization, we have localized central and peripheral nervous system expression of three transcripts (alpha1G, alpha1H, and alpha1I) of the T-type calcium channel family (CaVT). Each mRNA demonstrated a unique distribution, and expression of the three genes was largely complementary. We found high levels of expression of these transcripts in regions associated with prominent T-type currents, including inferior olivary and thalamic relay neurons (which expressed alpha1G), sensory ganglia, pituitary, and dentate gyrus granule neurons (alpha1H), and thalamic reticular neurons (alpha1I and alpha1H). Other regions of high expression included the Purkinje cell layer of the cerebellum, the bed nucleus of the stria terminalis, the claustrum (alpha1G), the olfactory tubercles (alpha1H and alpha1I), and the subthalamic nucleus (alpha1I and alpha1G). Some neurons expressed high levels of all three genes, including hippocampal pyramidal neurons and olfactory granule cells. Many brain regions showed a predominance of labeling for alpha1G, including the amygdala, cerebral cortex, rostral hypothalamus, brainstem, and spinal cord. Exceptions included the basal ganglia, which showed more prominent labeling for alpha1H and alpha1I, and the olfactory bulb, the hippocampus, and the caudal hypothalamus, which showed more even levels of all three transcripts. Our results are consistent with the hypothesis that differential gene expression underlies pharmacological and physiological heterogeneity observed in neuronal T-type calcium currents, and they provide a molecular basis for the study of T-type channels in particular neurons.
Figures





References
-
- Akaike N. T-type calcium channels in mammalian CNS neurones. Comp Biochem Physiol. 1991;98C:31–40. - PubMed
-
- Bhalla US, Bower JM. Exploring parameter space in detailed single neuron models: simulations of the mitral and granule cells of the olfactory bulb. J Neurophysiol. 1993;69:1948–1965. - PubMed
-
- Catterall WA. Structure and function of voltage-gated ion channels. Annu Rev Biochem. 1995;64:493–531. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
