Cloning and expression of a novel member of the low voltage-activated T-type calcium channel family
- PMID: 10066244
- PMCID: PMC6782566
- DOI: 10.1523/JNEUROSCI.19-06-01912.1999
Cloning and expression of a novel member of the low voltage-activated T-type calcium channel family
Abstract
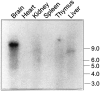
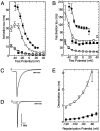
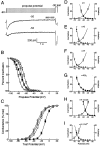
Low voltage-activated Ca2+ channels play important roles in pacing neuronal firing and producing network oscillations, such as those that occur during sleep and epilepsy. Here we describe the cloning and expression of the third member of the T-type family, alpha1I or CavT.3, from rat brain. Northern analysis indicated that it is predominantly expressed in brain. Expression of the cloned channel in either Xenopus oocytes or stably transfected human embryonic kidney-293 cells revealed novel gating properties. We compared these electrophysiological properties to those of the cloned T-type channels alpha1G and alpha1H and to the high voltage-activated channels formed by alpha1Ebeta3. The alpha1I channels opened after small depolarizations of the membrane similar to alpha1G and alpha1H but at more depolarized potentials. The kinetics of activation and inactivation were dramatically slower, which allows the channel to act as a Ca2+ injector. In oocytes, the kinetics were even slower, suggesting that components of the expression system modulate its gating properties. Steady-state inactivation occurred at higher potentials than any of the other T channels, endowing the channel with a substantial window current. The alpha1I channel could still be classified as T-type by virtue of its criss-crossing kinetics, its slow deactivation (tail current), and its small (11 pS) conductance in 110 mM Ba2+ solutions. Based on its brain distribution and novel gating properties, we suggest that alpha1I plays important roles in determining the electroresponsiveness of neurons, and hence, may be a novel drug target.
Figures



References
-
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. - PubMed
-
- Bech-Hansen NT, Naylor MJ, Maybaum TA, Pearce WG, Koop B, Fishman GA, Mets M, Musarella MA, Boycott KM. Loss-of-function mutations in a calcium-channel α1-subunit gene in Xp11.23 cause incomplete X-linked congenital stationary night blindness. Nat Genet. 1998;19:264–267. - PubMed
-
- Catterall WA. Structure and function of voltage-gated ion channels. Annu Rev Biochem. 1995;64:493–531. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
