The synaptophysin-synaptobrevin complex: a hallmark of synaptic vesicle maturation
- PMID: 10066245
- PMCID: PMC6782579
- DOI: 10.1523/JNEUROSCI.19-06-01922.1999
The synaptophysin-synaptobrevin complex: a hallmark of synaptic vesicle maturation
Abstract
Exocytosis of synaptic vesicles requires the formation of a fusion complex consisting of the synaptic vesicle protein synaptobrevin (vesicle-associated membrane protein, or VAMP) and the plasma membrane proteins syntaxin and soluble synaptosomal-associated protein of 25 kDa (or SNAP 25). In search of mechanisms that regulate the assembly of the fusion complex, it was found that synaptobrevin also binds to the vesicle protein synaptophysin and that synaptophysin-bound synaptobrevin cannot enter the fusion complex. Using a combination of immunoprecipitation, cross-linking, and in vitro interaction experiments, we report here that the synaptophysin-synaptobrevin complex is upregulated during neuronal development. In embryonic rat brain, the complex is not detectable, although synaptophysin and synaptobrevin are expressed and are localized to the same nerve terminals and to the same pool of vesicles. In contrast, the ability of synaptobrevin to participate in the fusion complex is detectable as early as embryonic day 14. The binding of synaptoporin, a closely related homolog of synaptophysin, to synaptobrevin changes in a similar manner during development. Recombinant synaptobrevin binds to synaptophysin derived from adult brain extracts but not to that derived from embryonic brain extracts. Furthermore, the soluble cytosol fraction of adult, but not of embryonic, synaptosomes contains a protein that induces synaptophysin-synaptobrevin complex formation in embryonic vesicle fractions. We conclude that complex formation is regulated during development and is mediated by a posttranslational modification of synaptophysin. Furthermore, we propose that the synaptophysin-synaptobrevin complex is not essential for exocytosis but rather provides a reserve pool of synaptobrevin for exocytosis that can be readily recruited during periods of high synaptic activity.
Figures

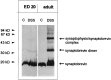






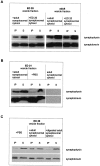
References
-
- Ahnert-Hilger G, Kutay U, Chahoud I, Rapoport TA, Wiedenmann B. Synaptobrevin is essential for secretion but not for the development of synaptic processes. Eur J Cell Biol. 1996;70:1–11. - PubMed
-
- Barnstable CJ, Hofstein R, Akagawa K. A marker of early amacrine cell development in rat retina. Brain Res. 1985;352:286–290. - PubMed
-
- Bauerfeind R, Huttner WB (1993) Biogenesis of constitutive secretory vesicles, secretory granules and synaptic vesicles. Curr Opin Cell Biol [Erratum 5:1106] 5:628–635. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
