Akt-dependent potentiation of L channels by insulin-like growth factor-1 is required for neuronal survival
- PMID: 10066247
- PMCID: PMC6782565
- DOI: 10.1523/JNEUROSCI.19-06-01940.1999
Akt-dependent potentiation of L channels by insulin-like growth factor-1 is required for neuronal survival
Abstract
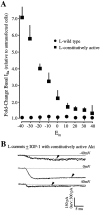
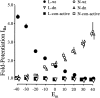
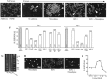
The insulin-like growth factor-1 (IGF-1)/receptor tyrosine kinase recently has been shown to mediate neuronal survival and potentiate the activity of specific calcium channel subtypes; survival requires Akt, a serine/threonine kinase. We demonstrate here that Akt mediates the IGF-1-induced potentiation of L channel currents, but not that of N channels. Transient expression of wild-type, dominant-negative, and constitutively active forms of Akt in cerebellar granule neurons causes, respectively, no change in IGF-1/L channel potentiation, complete inhibition of potentiation, and a dramatic increase in basal L currents accompanied by the loss of ability to induce further increases. In no case is the IGF-1 potentiation of N currents affected. We additionally find that IGF-1 partially mediates granule neuron survival via L channel activity and that Akt-dependent L channel modulation is a necessary component. Interestingly, very brief exposure (1 min) to IGF-1 triggers nearly complete survival and requires L channel activity. These results strongly suggest that neuronal receptor tyrosine kinases can control long-term calcium-dependent processes via the rapid control of voltage-sensitive channels.
Figures



References
-
- Alessi DR, James SR, Downes CP, Holmes AB, Gaffney PR, Reese CB, Cohen P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Curr Biol. 1997;7:261–269. - PubMed
-
- Andjelkovic M, Alessi DR, Meier R, Fernandez A, Lamb NJ, Frech M, Cron P, Cohen P, Lucocq JM, Hemmings MA. Role of translocation in the activation and function of protein kinase B. J Biol Chem. 1997;272:31515–31524. - PubMed
-
- Bean BP. Whole-cell recording of calcium channel currents. Methods Enzymol. 1992;207:181–193. - PubMed
-
- Blair LAC, Marshall J. IGF-1 modulates N and L calcium channels in a PI 3-kinase-dependent manner. Neuron. 1997;19:421–429. - PubMed
-
- Blair LAC, Bence KK, Marshall J. The jellyfish green fluorescent protein: a tool for studying ion channels and second messenger signalling in neurons. Methods Enzymol. 1999;302:213–225. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
