Cellular mechanisms contributing to response variability of cortical neurons in vivo
- PMID: 10066274
- PMCID: PMC6782570
- DOI: 10.1523/JNEUROSCI.19-06-02209.1999
Cellular mechanisms contributing to response variability of cortical neurons in vivo
Abstract
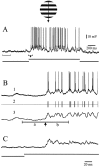
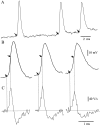
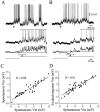
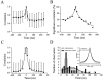
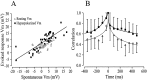
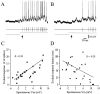
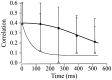
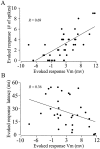
Cortical neurons recorded in vivo exhibit highly variable responses to the repeated presentation of the same stimulus. To further understand the cellular mechanisms underlying this phenomenon, we performed intracellular recordings from neurons in cat striate cortex in vivo and examined the relationships between spontaneous activity and visually evoked responses. Activity was assessed on a trial-by-trial basis by measuring the membrane potential (Vm) fluctuations and spike activity during brief epochs immediately before and after the onset of an evoked response. We found that the response magnitude, expressed as a change in Vm relative to baseline, was linearly correlated with the preceding spontaneous Vm. This correlation was enhanced when the cells were hyperpolarized to reduce the activation of voltage-gated conductances. The output of the cells, expressed as spike counts and latencies, was only moderately correlated with fluctuations in the preceding spontaneous Vm. Spike-triggered averaging of Vm revealed that visually evoked action potentials arise from transient depolarizations having a rise time of approximately 10 msec. Consistent with this, evoked spike count was found to be linearly correlated with the magnitude of Vm fluctuations in the gamma (20-70 Hz) frequency band. We also found that the threshold of visually evoked action potentials varied over a range of approximately 10 mV. Examination of simultaneously recorded intracellular and extracellular activity revealed a correlation between Vm depolarization and spike discharges in adjacent cells. Together these results demonstrate that response variability is attributable largely to coherent fluctuations in cortical activity preceding the onset of a stimulus, but also to variations in action potential threshold and the magnitude of high-frequency fluctuations evoked by the stimulus.
Figures


References
-
- Abeles M. Role of the cortical neuron: integrator or coincidence detector? Isr J Med Sci. 1982;18:83–92. - PubMed
-
- Abeles M, Bergman H, Margalit E, Vaadia E. Spatiotemporal firing patterns in the frontal cortex of behaving monkeys. J Neurophysiol. 1993;70:1629–1638. - PubMed
-
- Aertsen A, Preissel H. Dynamics of activity and connectivity in physiological neuronal networks. In: Schuster HG, editor. Nonlinear dynamics and neuronal networks. VCH Verlag; Weinheim, Germany: 1991. pp. 281–301.
-
- Aertsen A, Gerstein GL, Habib MK, Palm G. Dynamics of neuronal firing correlation: modulation of “effective connectivity.”. J Neurophysiol. 1989;61:900–917. - PubMed
-
- Aertsen AM, Smolders JW, Johannesma PI. Neural representation of the acoustic biotope: on the existence of stimulus-event relations for sensory neurons. Biol Cybern. 1979;32:175–185. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
