Bacillus subtilis spore coat
- PMID: 10066829
- PMCID: PMC98955
- DOI: 10.1128/MMBR.63.1.1-20.1999
Bacillus subtilis spore coat
Abstract
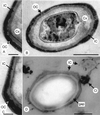
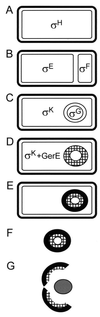
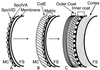
In response to starvation, bacilli and clostridia undergo a specialized program of development that results in the production of a highly resistant dormant cell type known as the spore. A proteinacious shell, called the coat, encases the spore and plays a major role in spore survival. The coat is composed of over 25 polypeptide species, organized into several morphologically distinct layers. The mechanisms that guide coat assembly have been largely unknown until recently. We now know that proper formation of the coat relies on the genetic program that guides the synthesis of spore components during development as well as on morphogenetic proteins dedicated to coat assembly. Over 20 structural and morphogenetic genes have been cloned. In this review, we consider the contributions of the known coat and morphogenetic proteins to coat function and assembly. We present a model that describes how morphogenetic proteins direct coat assembly to the specific subcellular site of the nascent spore surface and how they establish the coat layers. We also discuss the importance of posttranslational processing of coat proteins in coat morphogenesis. Finally, we review some of the major outstanding questions in the field.
Figures

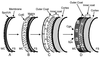
Similar articles
-
Maximum shields: the assembly and function of the bacterial spore coat.Trends Microbiol. 2002 Jun;10(6):251-4. doi: 10.1016/s0966-842x(02)02373-9. Trends Microbiol. 2002. PMID: 12088650 Review.
-
Structure, assembly, and function of the spore surface layers.Annu Rev Microbiol. 2007;61:555-88. doi: 10.1146/annurev.micro.61.080706.093224. Annu Rev Microbiol. 2007. PMID: 18035610 Review.
-
The coat morphogenetic protein SpoVID is necessary for spore encasement in Bacillus subtilis.Mol Microbiol. 2009 Nov;74(3):634-49. doi: 10.1111/j.1365-2958.2009.06886.x. Epub 2009 Sep 22. Mol Microbiol. 2009. PMID: 19775244 Free PMC article.
-
Coordinated Assembly of the Bacillus anthracis Coat and Exosporium during Bacterial Spore Outer Layer Formation.mBio. 2018 Nov 6;9(6):e01166-18. doi: 10.1128/mBio.01166-18. mBio. 2018. PMID: 30401771 Free PMC article.
-
Characterization of the Bacillus subtilis spore morphogenetic coat protein CotO.J Bacteriol. 2005 Dec;187(24):8278-90. doi: 10.1128/JB.187.24.8278-8290.2005. J Bacteriol. 2005. PMID: 16321932 Free PMC article.
Cited by
-
A gene encoding a holin-like protein involved in spore morphogenesis and spore germination in Bacillus subtilis.J Bacteriol. 2005 Sep;187(18):6443-53. doi: 10.1128/JB.187.18.6443-6453.2005. J Bacteriol. 2005. PMID: 16159778 Free PMC article.
-
Influence of glutamate on growth, sporulation, and spore properties of Bacillus cereus ATCC 14579 in defined medium.Appl Environ Microbiol. 2005 Jun;71(6):3248-54. doi: 10.1128/AEM.71.6.3248-3254.2005. Appl Environ Microbiol. 2005. PMID: 15933027 Free PMC article.
-
CotC-CotU heterodimerization during assembly of the Bacillus subtilis spore coat.J Bacteriol. 2008 Feb;190(4):1267-75. doi: 10.1128/JB.01425-07. Epub 2007 Dec 7. J Bacteriol. 2008. PMID: 18065538 Free PMC article.
-
In vitro studies of peptidoglycan binding and hydrolysis by the Bacillus anthracis germination-specific lytic enzyme SleB.J Bacteriol. 2011 Jan;193(1):125-31. doi: 10.1128/JB.00869-10. Epub 2010 Oct 22. J Bacteriol. 2011. PMID: 20971910 Free PMC article.
-
Label-free bacterial imaging with deep-UV-laser-induced native fluorescence.Appl Environ Microbiol. 2010 Nov;76(21):7231-7. doi: 10.1128/AEM.00943-10. Epub 2010 Sep 3. Appl Environ Microbiol. 2010. PMID: 20817797 Free PMC article.
References
-
- Abe A, Koide H, Kohno T, Watabe K. A Bacillus subtilis spore coat polypeptide gene, cotS. Microbiology. 1995;141:1433–1442. - PubMed
-
- Abe A, Ogawa S, Kohno T, Watabe K. Purification of Bacillus subtilis spore coat protein by electrophoretic elution procedure and determination of NH2-terminal amino acid sequences. Microbiol Immunol. 1993;37:809–812. - PubMed
-
- Alper S, Duncan L, Losick R. An adenosine nucleotide switch controlling the activity of a cell type-specific transcription factor in B. subtilis. Cell. 1994;77:195–206. - PubMed
-
- Arigoni F, Pogliano K, Webb C D, Stragier P, Losick R. Localization of protein implicated in establishment of cell type to sites of asymmetric division. Science. 1995;270:637–640. - PubMed
-
- Aronson A, Fitz-James P C. Biosynthesis of bacterial spore coats. J Mol Biol. 1968;33:199–212. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

