Metabolic engineering of poly(3-hydroxyalkanoates): from DNA to plastic
- PMID: 10066830
- PMCID: PMC98956
- DOI: 10.1128/MMBR.63.1.21-53.1999
Metabolic engineering of poly(3-hydroxyalkanoates): from DNA to plastic
Abstract
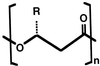
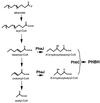
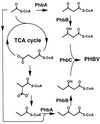
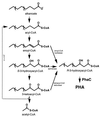
Poly(3-hydroxyalkanoates) (PHAs) are a class of microbially produced polyesters that have potential applications as conventional plastics, specifically thermoplastic elastomers. A wealth of biological diversity in PHA formation exists, with at least 100 different PHA constituents and at least five different dedicated PHA biosynthetic pathways. This diversity, in combination with classical microbial physiology and modern molecular biology, has now opened up this area for genetic and metabolic engineering to develop optimal PHA-producing organisms. Commercial processes for PHA production were initially developed by W. R. Grace in the 1960s and later developed by Imperial Chemical Industries, Ltd., in the United Kingdom in the 1970s and 1980s. Since the early 1990s, Metabolix Inc. and Monsanto have been the driving forces behind the commercial exploitation of PHA polymers in the United States. The gram-negative bacterium Ralstonia eutropha, formerly known as Alcaligenes eutrophus, has generally been used as the production organism of choice, and intracellular accumulation of PHA of over 90% of the cell dry weight have been reported. The advent of molecular biological techniques and a developing environmental awareness initiated a renewed scientific interest in PHAs, and the biosynthetic machinery for PHA metabolism has been studied in great detail over the last two decades. Because the structure and monomeric composition of PHAs determine the applications for each type of polymer, a variety of polymers have been synthesized by cofeeding of various substrates or by metabolic engineering of the production organism. Classical microbiology and modern molecular bacterial physiology have been brought together to decipher the intricacies of PHA metabolism both for production purposes and for the unraveling of the natural role of PHAs. This review provides an overview of the different PHA biosynthetic systems and their genetic background, followed by a detailed summation of how this natural diversity is being used to develop commercially attractive, recombinant processes for the large-scale production of PHAs.
Figures











References
-
- Abe C, Taima Y, Nakamura Y, Doi Y. New bacterial copolyesters of 3-hydroxyalkanoates and 3-hydroxy-ω-fluoroalkanoates produced by Pseudomonas oleovorans. Polym Commun. 1990;31:404–406.
-
- Abe H, Doi Y, Fukushima T, Eya H. Biosynthesis from gluconate of a random copolyester consisting of 3-hydroxybutyrate and medium-chain-length 3-hydroxyalkanoates by Pseudomonas sp. 61-3. Int J Biol Macromol. 1994;16:115–119. - PubMed
-
- Ackermann J-U, Babel W. Growth-associated synthesis of poly(hydroxybutyric acid) in Methylobacterium rhodesianum as an expression of an internal bottleneck. Appl Microbiol Biotechnol. 1997;47:144–149.
-
- Akiyama M, Doi Y. Production of poly(3-hydroxyalkanoates) from α,ω-alkanedioic acids and hydroxylated fatty acids by Alcaligenes sp. Biotechnol Lett. 1993;15:163–168.
-
- Akiyama M, Taima Y, Doi Y. Production of poly(3-hydroxyalkanoates) by a bacterium of the genus Alcaligenes utilizing long-chain fatty acids. Appl Microbiol Biotechnol. 1992;37:698–701.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

